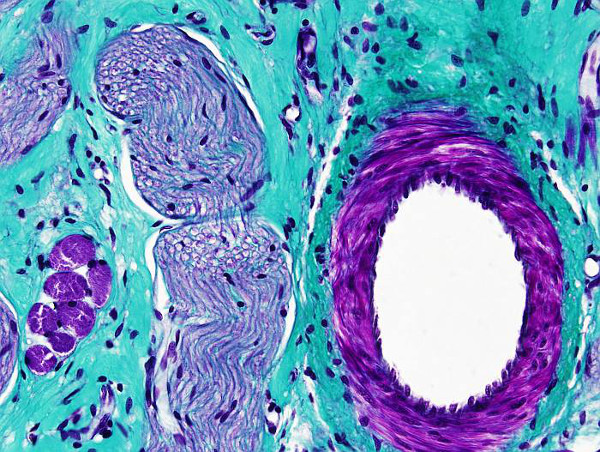
Trichrome means the three dyes used in this protocol: hematoxylin, fuchsin and light green. Masson trichrome is a very useful staining for distinguish collagen fibers, and connective tissue in general, from muscle fibers and epithelium. It is used for tumoral processes diagnosis. There are many variations of this staining protocol, which have been adapted to particular needs.
Procedure
Protocol adapted by Serxio Fernández Fidalgo.
Samples are fixed in Bouin and paraffin embedded. 8 µm thick sections are obtained and attached to gelatin coated slides.
1. 2x10 min xylene
2. 2x10 min 100º ethanol
3. 10 min 96º ethanol
4. 10 min 80º ethanol
5. 10 min 50º ethanol
6. 5 min distilled H2O
If tissues are not fixed in Bouin, it is a good practice to incubate sections in Bouin fixative for 24 h at room temperature, or 1 h at 56 - 60 ºC. Bouin works as mordant.te, durante 24 h a temperatura ambiente o 1 h a 56 - 60 ºC.
7. 3x3 min distilled H2O
8. 5 min Weigert hematoxylin (10 min if the staining solution is more than 5 days old).
9. 5 min tap water for differentition
The staining process can be checked at the microscope. If the color is not strong enough, sections can go back to Weigert hematoxylin.
10. 3 min rinse in distilled H2O
11. 5 min scarlet-fuchsin
Scarlet-fuchsin:
90 ml of 1% of Biebrich scarlet (C.I. 26905), diluted in distilled H2O.
9 ml of 1 % of acid fuchsin (C.I. 42685), diluted distilled in H2O
1 ml of glacial acetic acid
12. 2 min distilled H2O
In this step, the process of staining can be checked. However, keep in mind that differentiation is done is a forward step. If the color is weak, sections can go back to scarlet-fuchsin solution. If the color is too strong, sections can stay longed in distilled H2O.
13. 15 min 5 % of phosphomolibdic acid 5% diluted in distilled H2O
This step is necessary for a proper light green dye staining. The phosphomolibdic solution has to be fresh, and it should not be used many times. Fuchsin and scarlet is also removed in this step, and therefore it must be considered during previous staining processes checks. The sections can go back to previous steps in the color is lost.
14. 10 min 2 % light green (CI 42095) (it can be replaced by anline blue)
2 g Light green (CI 42095), 2 ml glacial acetic acid and 98 ml distilled H2O
15. several seconds in distilled H2O
The staining quality can be checked under the microscope. If it is not good enough, sections can go back and be stained for a longer time in the light green dye, but always with a previous step in phosphomolibdic acid. The dehydration steps with acidic water and 96 º ethanol can be extended if the staining is too strong.
16. 3 min 1% glacial acetic acid in distilled H2O. Differentiation.
1 min is enough if the sections are not strongly stained. More time may fade out the color from the tissues. demasiado.
17. Quick dehydration. Several seconds in 80º, 96º y 100º ethanol
The 96 º ethanol step has to be no longer than a few seconds because the light green staining progressively disappears. At the same time, scarlet-fuchsin staining changes a bit to a more light and pinky color. A very good staining depends a lot on properly adjusting the time in this step. If the staining is not good enough, the slides can go to previous steps of the protocol by re-hydrating the tissues.
18. 2x10 min xylene.
19. Mounting and coversliped.
Resultados
Collagen: blue-green
Muscle: red-brown
Cytoplasm: pink
Nucleus: black
Notes
Sections can go back to previous steps to improve the quality of the staining, but no more than 3 o 4 times because the tissue progressively loses the capacity to retain the color.
Products
Xylene
50º, 70º, 80º, 90º, 96º y 100º ethanol
Weigert hematoxylin
Biebrich scarlet (C.I. 26905)
Acid fuchsin (C.I. 42685)
5 % phosphomolibdic acid
Light green (CI 42095)
Distillled H2O
Tap water
Mounting medium
Labware
Stining dishes
Slides racks
Coverslips