The endoplasmic reticulum mostly communicates with the Golgi apparatus by vesicular trafficking. Both organelles are the first and second stages of the secretory pathway, respectively. Most proteins and lipids leaving the endoplasmic reticulum are enclosed in vesicles or are components of the vesicular membrane (Figure 1). They are delivered to the Golgi apparatus. In plants, however, there is an alternative pathway toward the vacuole and even to the plasma membrane.
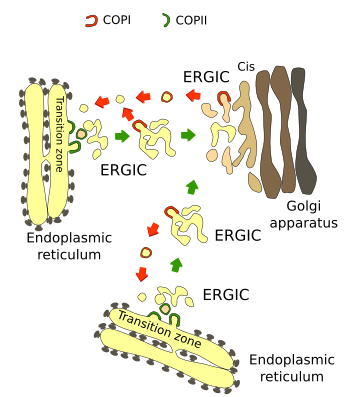
Vesicles are formed and depart from specialized regions called reticular exit zones (or transitional regions). These zones are numerous, ranging from 50 to hundreds in one typical mammalian cell, they lack ribosomes and are scattered through the membranes of the endoplasmic reticulum. Exit zones are about 0.5 µm in length and quite stable in both position and size. They are associated with stacks of Golgi cisterns, that is, both are often found close to each other. It makes sense because the efficiency of the transport between both organelles is improved: vesicles have to travel shorter distances, and the Golgi stacks are more stable since the Golgi apparatus needs material from the endoplasmic reticulum for both maintaining its function and morphological integrity. It has been observed that when a new exit zone is formed, a new Golgi stack appears nearby, and the opposite occurs if an exit zone disappears, the associated Golgi stack is no longer present. Sometimes, two close exit zones may fuse together, or one zone may be split in two, and the same behavior is observed in the associated Golgi stacks.
COPII coating proteins are involved in the formation of vesicles in the endoplasmic reticulum (Figure 1). This coat consists of Sec12/16, Sar1 GAPase, Sec 23/24 and Sec13/31. They are assembled on the surface of the vesicle in this order. The endoplasmic reticulum exit zones form a suitable environment for the COPII assembly: a distinct lipid composition and many associated Sec12/16 proteins, which recruit Sec23/24, which in turn recruit Sec13/36. COPII proteins perform two functions: they are involved in the vesicle formation (mainly the outer layer composed of Sec13/36) and cooperate in the cargo section.
Cargoes are proteins and lipids to be included in COPII-coated vesicles. Selected proteins have amino acid sequences, known as exportation sequences, that are recognized by receptors. There are soluble and integral membrane proteins that have to be transported. Soluble proteins are "caught" by membrane receptors, whereas integral membrane proteins (i.e., transmembrane proteins) are directly recognized by coat proteins. Both receptor types need to interact with the COPII proteins. Sec24 recognizes exportation sequences. However, since there are a variety of cargoes, there are also subtypes of Sec24 proteins. Up to 13 cargo receptors have been found in yeast and up to 24 in humans. The ERGIC-53 protein moves between the endoplasmic reticulum and the Golgi apparatus and shows sequences for both COPII and COPI.
Aunque las cargas transmembrana son reconocidas directamente por las proteínas de la cubierta a través de su dominio citosólico, hay cada vez más datos que indican que algunas proteínas transmembrana son reconocidas por otras proteínas transmembrana, como las proteínas denominada cornichon. Las proteínas cornichon tienen tres cruces de membrana y secuencias en sus dominios citosólicos para COPII y para COPI. Los receptores acoplados a proteínas G (proteínas transmembrana) necesitan a cornichon para salir del ER.
Although many transmembrane proteins are directly recognized by coat proteins by their cytosolic domain, there is an increasing amount of evidences suggesting that some transmembrane proteins are recognized by other transmembrane proteins, such as cornichon proteins. These proteins have an amino acid chain that crosses the membrane three times, and their cytosolic domains interact with COPII and COPI. The G protein-coupled receptors (GPCR) are exported from the endoplasmic reticulum with the help of cornichon proteins.
COPII proteins select cargoes to be included in vesicles. However, some molecules can be unspecifically enclosed during vesicle budding. This way of exiting the endoplasmic reticulum is referred to as bulk flow. It is estimated that about half of the endoplasmic reticulum luminal fluid is enclosed in COPII vesicles every 40 minutes, which means that about 150 vesicles are formed per second. Nonetheless, there are mechanisms to minimize the loss of internal content. For example, it has been observed that there are high-density protein aggregates that favor the molecular interactions, hinder lateral diffusion and hence the chance to access the exit zones is lower. Another mechanism may work for resident transmembrane proteins. They do not fit well in the vesicle membrane because they have shorter fatty acid chains. It is interesting that when foreign proteins are expressed in the endoplasmic reticulum, there is no problem with their export by means of vesicles. The pharmaceutical industry takes advantage of this process to get some useful proteins from eukaryotic cultures.
Although the transport from the endoplasmic reticulum to the Golgi apparatus is largely done by COPII vesicles, there are alternatives for carrying some molecules between both organelles. For instance, a typical COPII vesicle is 60 to 90 nm in diameter, but a procollagen molecule is about 300 to 400 nm long. Chylomicrons are also larger than COPII vesicles. It seems that the GTPase Sar1 is involved in the modification of the COPII assembly to make larger vesicles to accommodate these larger charges.
No matter the transport pathway, proteins to be exported need to be properly folded to get a precise spatial conformation. Misfolded proteins have to be removed before they reach the export machinery. In the endoplasmic reticulum, there is a quality control for detecting errors in the 3D conformation of proteins. This mechanism is called ERAD (endoplasmic reticulum-associated protein degradation).
COPII coated vesicles released from the endoplasmic reticulum loss part of the coat and fuse to one another to form the ERGIC, which is moved by the cytoskeleton to the cis domain of the Golgi apparatus. The ERGICs become the first cisterns of the Golgi apparatus.
The partial uncoating of COPII vesicles favors the release of some cargoes from their receptors. Other cargoes are freed in the cis domain of the Golgi apparatus. Part of the vesicle content is recycled back to the endoplasmic reticulum enclosed in COPI coated vesicles or through transient tubules formed between both organelles. These tubules are mediated by Rab6 proteins. COPI vesicles may bud off from the both the ERGIC and the Golgi apparatus. They are nucleated in membrane regions with a high content of Arf1 proteins. This bidirectional trafficking has two roles: to send back resident proteins and to keep the sizes of both organelles relatively constant.For instance, it has been estimated that every 1 or 2 hours, a mammalian cell exports all the endoplasmic reticulum membrane in COPII vesicles. The ERGIC progressively changes on its way toward the Golgi apparatus. It is a kind of maturation where the set of membrane proteins is modified, allowing the recruitment of COPI proteins. COPI is stabilized by the cargoes that have to be recycled, so the more cargoes for recycling, the more COPI vesicles are formed.
The cargo selection by COPI vesicles depends on sequences of amino acids in the carboxyl terminal of the protein. They are known as retrieval sequences, which are recognized by COPI. KDEL receptors recognize these sequences, which are found in many resident proteins of the endoplasmic reticulum. KDEL alternates between the endoplasmic reticulum and the ERGIC/Golgi apparatus, either linked to its cargo (Golgi/ERGIC to the endoplasmic reticulum) or free (endoplasmic reticulum to the Golgi/ERGIC). The affinity between the KDEL and its cargo is affected by the pH. Lower pH increases the affinity. Hence, the cargoes are recognized in the more acidic ERGIC/Golgi apparatus and freed in the more basic endoplasmic reticulum. Similarly to KDEL, Rer1 andn Erv41-Erv46 are other receptors that bind endoplasmic reticulum transmembrane resident proteins in the ERGIC/Golgi apparatus membranes, but by recognizing other amino acid sequences different from KDEL sequences.
COPI coated vesicles have to carry back to the Golgi apparatus enough membrane to replace the membrane used to form COPII vesicles, as well as resident proteins of the endoplasmic reticulum and receptors, and all the machinery for recognizing and fusing with the endoplasmic reticulum.
-
Bibliography ↷
-
Bibliography
Antonny B, Schekman R. 2001. ER export: public transportation by the COPII coach. Current opinion in cell biology. 13:438-443.
Barlow C, Helenius A. 2016. Cargo capture and bulk flow in the early secretory pathway. Annual review of cell and developmental biology. 32: 197-222.
Saito K, Maeda M, Katada T. 2017. Regulation of the Sar1 GTPase cycle is necessary for large cargo secretion from the endoplasmic reticulum. Frontiers in cell and development biology. 5: 75.
Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. 2012. COPII and the regulation of protein sorting in mammals. Nature cell biology. 14: 20-28.
-
 Endoplasmic reticulum
Endoplasmic reticulum 