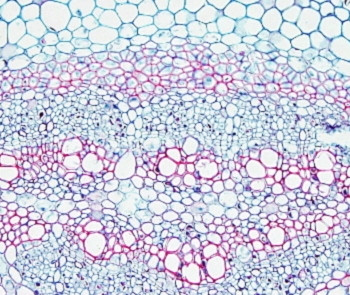
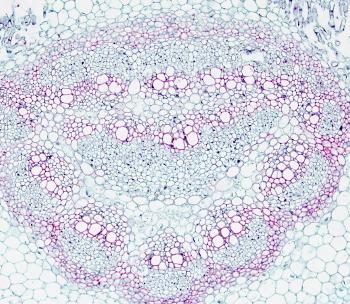
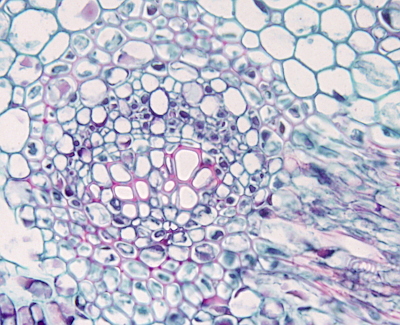
In plant tissues, staining protocols, besides staining nuclei and cytoplasm, are aimed to stain cell walls. Common plant tissues staining use safranin to stain lignified cell walls (mostly secondary walls) and other dye, such as Alcian blue or fast green, to stain non-lignified cell walls (primary cell walls). Johansen's safranina combined with fast green is a good staining for the study of plant tissues.
Procedure
Plant samples fixed with FAA (formaldehyde, acetic acid, alcohol) are embedded in paraffin and sectioned in 5 to 10 µm thick sections. Vibratome and freezing microtome sections can be uses as well, but the times in each step have to be dajusted.
1.- 2x10 min in xylene
2.- 2x10 min in 100º ethanol
3.- 10 min in 96º ethanol
4.- 10 min in 80º ethanol
5.- 10 min in 50º ethanol
6.- 5 min in distilled H2O
7.- 24 h in Johansen's O safranin
Johansen's O safranin
2-methoxyethanol (Ethylene glycol monomethyl ether): 50 ml
100º ethanol: 25 ml
Distilled H2O: 25 ml
Sodium acetate: 1 g
Dissolve
Formaldehyde 37 % (formalin): 2 ml
Safranin O (C.I. 50240): 1 g
8.- 2 x 5 min in destilled H2O
9.- 10 s in 96º ethanol + 0.5 % picric acid(or clorhydric acid) with gentle agitation.
10.- 10 s in 96º ethanol + 4 drops of ammonium hydroxide with gentle agitation.
11.- 10 s in 100º ethanol with gentle agitation.
Safranin staining is regressive, so that the intensity of the red color is set with the three steps after the dye. If they are too long, the red color may be dull or disappear.
12.- 20 s in fast green with gentle agitation.
Fast green
2-methoxyethanol (Ethylene glycol monomethyl ether): 33.3 ml
100º ethanol: 33.3 ml
Methyl salicylate: 33.3 ml
Fast green FCF (C.I. 42053): 0.05 g
13.- 5 a 10 s in diluted clearing solution with gentle agitation.
Diluted clearing solution
Clearing solution (see below): 50 ml
100º ethanol: 25 ml
Xylen: 25 ml
14.- 10 s in clearing solution with gentle agitation.
Clearing solution
Methyl salicylate: 50 ml
100º ethanol: 25 ml
Xylene: 25 ml
15.- 2x10 min in xylene
16.- Mounting medioum and coverslip.
Notes
The length of the steps after the dyes determines the quality of the staining.
Safranin staining is regressive, so that the color intensity is controlled with the following ethanol clearing steps, whereas the fast green stain is progressive, that is, the color intensity depends on the time in the dye.
Since a gentle agitation of the slides (and the attached sections) is recommended for a better washing and clearing of sections, it is sometimes advisable to do the staining procedure slide by slide.
Products
Xylene
50º, 70º, 90º and 100º ethanol
Sodium acetate
Chlorhydric acid
Safranin O (C.I. 50240)
Formaldehyde 37%
2-methoxyethanol
Methyl salicylate
Fast green FCF (C.I. 42053)
Picric acid
Ammonium hydroxide
Distilled H2O
Mounting medium
Labware
Staining dishes
Forceps
Slides
Coverslps
Containers for the solutions