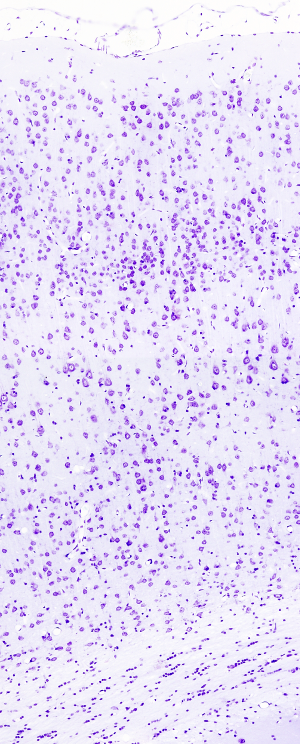
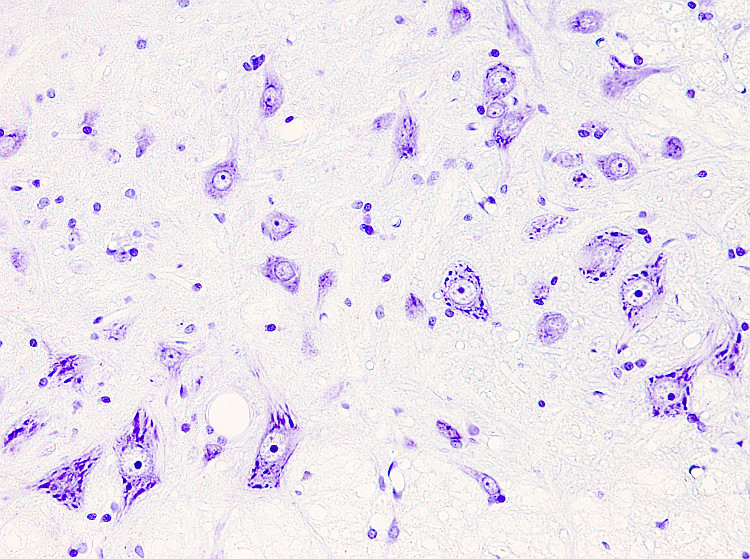
Nissl staining is widely used to study the organization of neurons in nuclei and layers throughout the nervous system, mostly brain and spinal cord. However, other tissues can be stained. Toluidine blue or Cresyl violet can be the dyes present in the procedure. Cresyl violet is more often used, which is the procedure described below.
In the neuron cytoplasm, strongly stained structures are visible after Nissl staining. They are referred to as Nissl bodies, which correspond with rough endoplasmic reticulum aggregates. Ribosomal RNA and messenger ARN, which is being translated, are the acidic molecules stained in the Nissl bodies.
Procedure
Samples are fixed with formaldehyde (Bouin or 4 % paraformaldehyde) and embedded in paraffin. 5 to 10 µm thick sections are obtained and attached to gelatin coated slides.
1.- 2x10 min in xylene
2.- 2x10 min in 100º ethanol
3.- 10 min in 96º ethanol
4.- 10 min in 80º ethanol
5.- 10 min in 50º ethanol
6.- 5 min in distilled H2O
7.- 5-10 min in 0.1 % of crsyl violet solution(CAS: 10510-54-0)
There are several ways for preparing cresyl violet solution:
a) 0.1 g of cresyl violet in 100 ml of distilled H2O. Just before use, add a few drops of glacial acetic acid.
b) 0.1 g of cresyl violet in 100 ml of distilled H2O. Add 0.25 ml of glacial acetic acid. Stir the solution at 60 ºC until the dye is completely dissolved. Store the solution in a dark place and filter it before use.
c) Acetate buffer solution: solution A: 0.6 ml acetic acid in 100 ml of distilled H2O. Solution B: 1.36 g sodium acetate in 100 ml of distilled H2O. Mix solutions A and B in a proportion 9:1 (9 ml of A and 1 ml of B), and adjust the pH to 3.7. Mix 1 % of cresyl violet dissolved in H2O and acetate buffer solution in a proportion 1:1. Filter it before use.
For thick sections, the staining solution can be warmed up to 37 ºC.
8.- Quick wash in distilled H2O
9.- Differentiate in 96º ethanol for several min. Check the process under the microscope.
10.- 2x10 min in 100º ethanol
11.- 2x10 min in xylene
15.- Mounting and coversliped.
Results
Nuclei: purple - violet.
Rough endoplasmic retiuculum: purple - violet.
Notes
The quality of the staining depends on the differentiation time during the dehydration process, particularly in the 96º ethanol.
Lipid droplets or myelin (vibratome, cryostate, freezing microtome sections) may affect the staining of the cell bodies. These sections may be dehydrated in ethanol to dissolve any lipid and re-hydrated again.
Products
Xylene
50º, 70º, 80º, 90º, 96º y 100º ethanol
Cresyl violet (CAS: 10510-54-0)
Acetic acid
Sodium acetate
Distilled H2O
Mountin medium
Labware
Staining dishes
Filter paper
Test tube
Small jars
Slide racks
Coverslips