This is a summary of the final Biology degree project by Clara Leboreriro Babé, 2017, University of Vigo.
Many pathologies are identified and evaluated by using immunohistochemical techniques. The quality of the results of these techniques are often essential to take a good decision about the treatment or to establish the progression of the disease. One important aspect of immunohistochemical techniques is to realize that the antigens to be recognized by the antibodies may be altered or damaged during fixation and further histological processing.
In most clinical pathology labs, samples are fixed with 4% formaldehyde and embedded in paraffin. Formaldehyde produces many and complex reactions between the proteins in the tissues, mainly cross-linking (Figure 1). A long fixation leads to damages in many protein domains that are antigen determinants (epitopes), recognized by antibodies during immunostaining. The cross-linking produced by the fixative changes the tridimensional arrangement of proteins, which may hide or mask the antigen determinants so that antibodies can not "find" them. More than 24 hours of fixation leads to over-fixed samples. In aqueous solutions, formaldehyde forms hydrate ethylene, which reacts with many lateral chains of proteins and forms hydroxymethyl reactive groups. These groups link other proteins by methylene bridges. The most reactive lateral chains of proteins are those containing cysteine, lysine, histidine and tyrosine amino acids.
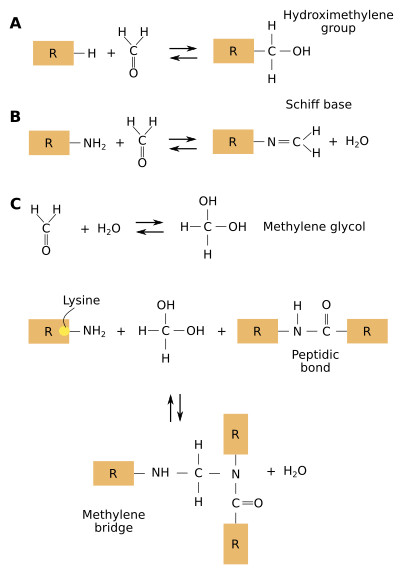
In immunohistochemistry techniques, the target proteins for the antibodies can be cross-linked to other proteins. This cross-bindings may hinder the recognition of the antigen determinants by the antibody because the recognition sites are hidden or masked. Therefore, false negative results may be produced. This means that the technique suggests that there is no a particular type of protein in the tissue, but it is actually there, although not detected by the antibody.
In addition, formation of calcium complexes, changing the protein conformational arrangement, and alterations of the electric charges of proteins are tissue modifications caused by formaldehyde that may mask the antigens. It is thought that formaldehyde mostly affects the primary structure of proteins, but it is less active in changing the secondary and tertiary order structure of proteins. However, some alterations of the tertiary structure may happen, therefore affecting the conformational epitopes. The antigen-antibody recognition depends on electric charges, and fixation with formaldehyde can modify superficial charges of proteins that may affect the antigen determinant, preventing it to be recognized by the antibody.
The cross bindings formed by formaldehyde are stable at some pH and temperature ranges, and they also depend on the features of the solutions where the tissue is incubated or stored. These bindings can be broken by some treatments so that the antigen determinants are exposed again. This process of antigen unmasking is known as antigen retrieval. It is performed to revert the effect of fixation on proteins so that the antigen determinants recover the same features they have before fixation, and are exposed to be recognized by the antibodies.
The antigen retrieval methods have improved the immunohistochemistry as a diagnostic tool. Because of the impact of these methods in histopathology, two periods can be established in the history of immunohistochemistry: pre-antigen retrieval and post-antigen retrieval. Formaldehyde is routine fixation in the histopathology labs, as well as in the tissue banks. In these samples, an antigen retrieval method is the common step before the incubation with the antibodies. Additional advantages are: more diluted antibodies can be used because the threshold for antigen detection is lower, it is cheaper because more immunodetections can be done from an initial amount of antibody, the background staining is reduced, and the labeling is stronger. The samples of the tissue banks are becoming extremely valuable since very old samples, even stored for decades, can be processed for immunohistochemistry for clinical research thanks to the antigen retrieval methods. Immunohistochemistry can be combined with proteomic studies for searching biomarkers in the personalized medicine field.
Since cross-linking between amino acid chains of proteins during formaldehyde fixation depends on the primary structure of proteins, the amino acid sequence of the protein to be detected should be considered for adapting the antigen retrieval protocol. Some antigens need to be unmasked to be detected by immunohistochemistry, and some can be studied without antigen retrieval. However, unmasking protocols usually enhance the signal of the immunoreaction, no matter the antigen studied. The correct adjustment of the antigen retrieval protocol is important to prevent false positive and false negative results. A wrong protocol may even hide an antigen. Thus, it is recommended to try and choose the best antigen retrieval protocol for a particular antigen to get the best results.
1. Antigen retrieval methods
Initially, the antigen retrieval methods began with boiling the sections in water. Later, the water was substituted for buffered solutions to keep the conformational organization of proteins. Nowadays, there are a wide arrange of antigen retrieval methods that may combine difference heat sources, several buffered solutions and enzymatic activity. The unmasking by heat is the most widely used method. The enzymatic method is only chosen when the antigen determinant is lost by heating, such as cytokeratins. The more common enzymes used for unmasking antigens are trypsin, proteinase K, pepsin, pronase, and ficins.
There are two types of antigen retrieval methods: heat-induced epitope retrieval (HIER) and proteolytic-induced epitope retrieval (PIER).
Heating methods
The HIER method is the most widely used, with variations depending on the heat sources. This method has greatly improved the immunohistochemistry in the pathology labs because it has allowed the detection of a number of antigens that could not be previously studied. It is though that heating the tissue breaks the cross-linking caused by formaldehyde during fixation, although it has not been proved yet. Most HIER protocols use microwaves or an autoclave-like pressure cooker as heating sources. A hot bath or an oven can alternatively be used. Each heating source has advantages and shortcomings (see Table 1). It is curious that immunohistochemistry in alcohol fixated samples, which does not establish cross-bindings, is also improved by HIER methods.
Table 1. Summary of the main features of different heating sources (HIER):
| Hot bath | Microwaves | Pressure cooker | |
| Temperature rank | 25 to 100 ºC | 85 to 95 ºC | 25 to 125 ºC |
| Temperature regulation | Good | Good | Optimal |
| Buffer solution evaporation | Important | Important | Very low |
| Boiling overflow | No | Important | No |
The pressure cooker, or autoclave, is the most used heating apparatus. It can get both temperatures over 100 ºC and prevents boiling, thus shortening the treatment time. The heating time should be set for each type of sample, but 8 to 10 min at 110-120 °C is routinely used. However, the results may be slightly different with variations of 2 to 3 min of heating time, as well as different cooling times. It should mind that heating may increase the reactivity of the endogenous biotin, which may lead to false positive results. For example, the mitochondrial pyruvate carboxylase bears 4 biotin molecules, that can be labeled by biotin-avidin based immunohistochemistry in metabolically active cells. That is why it is important to perform a control of the immunohistochemistry technique by removing only the primary antibody. Solutions containing urea may enhance this endogenous biotin reactivity, while they are lower in Tris buffered solutions.
The microwave apparatus is another heating source. It heats the solution and sample very fast because the microwave heats the interior of the sample too, by heating the water within tissues. Thus, it is useful for large samples. The problem with the microwave apparatuses is that they have their own "personality", and standardizing the results should be done in each laboratory. In addition, the volume of the solutions affect the heating speed so that it has to be set for each volume of solution to be used. Another shortcoming of microwaves is the irregular heating through the solutions and samples, so that it is necessary a rotation of the container during heating. A visual control of the heating process has to be done to prevent sudden and strong boilings of the solution, leading to the replenishment of the solution, which alters the temperature values along the heating time.
The pH and temperature of the solution where the sample is heated are two main variables of the HIER method determining the efficiency of antigen retrieval. The higher temperature value and the heating time length have to be properly set. Thus, as the temperature is higher the heating time needs to be shorter, and the other way around. The composition of the solution where the sample is immersed may not be as important as the pH. For example, some antigens are best unmasked at basic pH, while others needs acid pH. Citrate buffer is routinely used for acidic pHs, whereas Tris buffer is selected for basic pH. Some examples of solutions are citrate buffer at pH 6, Tris buffer at pH 10, chloride-glycine 0.05 M, EDTA 0.1M at pH 8, Periodic acid 0.1 %, several concentrations of urea, lead thiocyanate, and some others.
Calcium may affect antigen retrieval because it seems to mask antigens by promoting the formation of molecular complexes during tissue processing. Thus, EDTA is added to the solutions in several antigen retrieval protocols. EDTA is a calcium chelator that removes free calcium. However, a real improvement of EDTA of antigen retrieval is controversial.
Enzymes
The enzymatic method (PIER) makes use of proteases, such as K protease and trypsin, to remove the methylene bridges formed during formaldehyde fixation. The length of incubation in the enzyme solution depends on the fixation time. Since proteases may damage the proteins of the tissue and therefore the antigen determinants, the enzymatic method is not widely used in histology labs. However, when the HIER methods do not properly unmask the antigens, a combination of HIER and PIER methods can be used.
2. Evaluation
The quantitative studies of the immunohistochemical labeling are not so frequent, and qualitative evaluation at light microscopy is usually performed. However, the digital image analysis allows a quantitative measurement of the results. These quantitative data depend on how the acquisition of the images is done with the microscope and on the image analysis program.
Image acquiring. The images may be taken in grayscale or in RGB range color. The compensatory effects of photography cameras, such as compensatory colors, illumination, white balance, as well as the lenses of the microscope, should be considered. In this way, all the images to be compared have to be taken in the same conditions.
There are two types of sensors in digital cameras, CCD (charge coupled device) and CMOS (complementary metal oxide semiconductor). CCD technology outmuch CMOS and it is more suitable for scientific analysis since the pixel size is larger. In addition, CMOS often produces more noise in the digital images, particularly at fluorescence microscopy. Images formats are also important because of the compression algorithms and the consequent loss of quality and information. The quality of the image also depends on the resolution or size. Therefore, a balance between the compression rate and the size of the image has to be set for a better quality during the image acquiring.
In a digital image analysis, the color intensity of the immunopositive structures has to be correlated with the amount of antigens, so that more intensity means more number of antigens. Even if the image processing program can adjust pictures for a proper quantification, the samples to be quantified and compared must accurately follow the same protocol: sample collection, fixation, embedding, antigen retrieval, and immunostaining. Otherwise, staining differences may be caused by slightly different protocols, which leads to incorrect results.
Bibliografía
Alelú-Paz, R., Haroutunian, V., Iturrieta-Zuazo, I., Byne, W., García-Villanueva, M., Rábano, A., García-Amado, M., Prensa, L., Giménez-Amaya, J.M. (2008). A new antigen retrieval technique for human brain tissue. PLOS ONE. 3: e3378.
Balgley, B.M., Guo, T., Zhao, K., Fang, X., Tavassoli, F.A., Lee, C.S. (2009). Evaluation of archival time on shotgun proteomics of formalin-fixed paraffin-embedded tissues. J. Proteome. Res. 8: 917-925.
Battifora, H. (1991). Assessment of antigen damage in immunohistochemistry. The vimentin internal control. Am. J. Clin. Pathol. 96: 669-671.
Boenisch, T. (2006). Heat-induced antigen retrieval: what are we retrieving? J. Histochem. Cytochem. 54: 961-964.
Bogen, S., Vani, K., Sompuram, S. (2009). Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced crosslinks. Biotech. Histochem. 84: 207-215.
D’Amico, F., Skarmoutsou, E., Stivala, F. (2009). State of the art in antigen retrieval for immunohistochemistry. J. Immunol. Methods. 341: 1-18.
Elias, J.M., Rosenberg, B., Margiotta, M., Kutcher, C. (1999). Antigen restoration of MIB-1 immunoreactivity in breast cancer: combined use of enzyme predigestion low temperature for improved measurement of proliferation indexes. J. Histotechnology. 22: 103-106.
Eltoum, I., Fredenburgh, J., Myers, R.B., Grizzle, W.E. (2001). Introduction to the theory practice of fixation of tissues. J. Histotechnology. 24: 173-190.
Ezaki, T. (2000). Antigen retrieval on formaldehyde-fixed paraffin sections: its potential drawbacks optimization for double immunostaining. Micron. 31: 639-649.
Floyd, A.D. (2010) Image analysis in inmunohistochemistry. En: Shi, S-R., Taylor, C.R. Antigen retrieval inmmunohistochemistry based research diagnostics. New Jersey: John Wiley & Sons Inc, pp. 165-185.
Fowler, C.B., Evers, D.L., O’Leary, T.J., Mason, J.T. (2011). Antigen retrieval causes protein unfolding. J. Histochem. Cytochem. 59: 366-381.
Fox, C.H., Johnson, F.B., Whiting, J., Roller, P.P. (1985). Formaldehyde fixation. J. Histochem. Cytochem. 33: 845-853.
Fraenkel-Conrat, H., Cooper, M., Olcott, H.S. (1945). The reaction of formaldehyde with proteins. J. Am. Chem. Soc. 67: 950-954.
Frost, A.R., Sparks, D., Grizzle, W.E. (2000). Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 8: 236-243.
Gown, A.M., Willingham, M.C. (2002). Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J. Histochem. Cytochem. 50: 449-454.
Gown, A.M. (2004). Unmasking the mysteries of antigen or epitope retrieval formalin fixation. Am. J. Clin. Pathol. 121: 172-174.
Kahveci, Z., Minbay, F., Noyan, S., Çavusoglu, I. (2003). A comparison of microwave heating proteolytic pretreatment antigen retrieval techniques in formalin fixed, paraffin embedded tissues. Biotech. Histochem. 78: 119-128.
Long, D.J., Buggs, C. (2008). Microwave oven-based technique for immunofluorescent staining of paraffin-embedded tissues. J. Mol. Histol. 39: 1-4.
Namimatsu, S., Ghazizadeh, M., Sugisaki, Y. (2005). Reversing the effects of formalin fixation with citraconic anhydride heat: a universal antigen retrieval method. J. Histochem. Cytochem. 53: 3-11.
Rait, V.K., O’Leary, T.J., Mason, J.T. (2004). Modeling formalin fixation antigen retrieval with bovine pancreatic ribonuclease A: I-Structural functional alterations. Lab. Invest. 84: 292-299.
Ramos-Vara, J.A., Beissenherz, M.E. (2000). Optimization of immunohistochemical methods using two different antigen retrieval methods on formalin-fixed paraffin-embedded tissues: experience with 63 markers. J. Vet. Diagn. Invest. 12: 307-311.
Shi, S.R., Cote, R.J., Taylor, C.R. (1997). Antigen retrieval immunohistochemistry: past, present, future. J. Histochem. Cytochem. 45: 327-343.
Shi, S.R., Cote, R.J., Taylor, C.R. (2001). Antigen retrieval techniques: current perspectives. J. Histochem. Cytochem. 49: 931-937.
Shi, S.R., Cote, R.J., Yang, C., Chen, C., Xu, H.J., Benedict, W.F., Taylor, C.R. (1996). Development of an optimal protocol for antigen retrieval: a “test battery” approach exemplified with reference to the staining of retinoblastoma protein (pRB) in formalin-fixed paraffin sections. J. Pathol. 179: 347-352.
Shi, S.R., Imam, S.A., Young, L., Cote, R.J., Taylor, C.R. (1995). Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J. Histochem. Cytochem. 43: 193-201.
Shi, S.R., Key, M.E., Kalra, K.L. (1991). Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 39: 741-748.
Shi, S.R., Shi, Y., Taylor, C.R. (2011). Antigen retrieval immunohistochemistry: review future prospects in research diagnosis over two decades. J. Histochem. Cytochem. 59: 13-32.
Taylor, C.R., Shi, S.R., Chen, C., Young, L., Yang, C., Cote, R.J. (1996). Comparative study of antigen retrieval heating methods: microwave, microwave pressure cooker, autoclave, steamer. Biotech. Histochem. 71: 263-270.
Yamashita S., Okada Y. (2005) Mechanisms of heat-induced antigen retrieval: analyses in vitro employing SDS-PAGE immunohistochemistry. J. Histochem. Cytochem. 53: 12-21.