1. Selection
- Pinocytosis
- Receptors
2. Types
- Clathrin
- Caveolae
- Non-coated
- Macropinocytosis
- Phagocytosis
It is essential for cell survival to catch external molecules and get them into the cell. Some molecules can cross the plasma membrane by passive diffusion, or mediated by channels, transporters or pumps. Endocytosis is a cell mechanism to get into the cell large amount of molecules or particles enclosed in plasma membrane that forms membrane bound compartments in the cytoplasm. These compartments filled with molecules are mostly vesicles, but also larger membrane bound compartments. The final target of these compartments are early endosomes. Vesicles fuse with early endosomes, and larger compartments become an early endosome by maturation.
Endocytosed molecules are mostly targeted for degradation in lysosomes in order to get energy and molecular building blocks for the cell metabolism. This is a main function of endocytosis. However, it also performs other functions. The plasma membrane is constantly renewed by removing lipids and proteins as part of the endocytotic vesicles. Endocytosis also balances the addition of new membrane to the plasma membrane carried by exocytosis, preventing an increase of the plasma membrane surface. Many receptors of the plasma membrane are included as part of the endocytic vesicle membranes, so that endocytosis may influence the amount of receptor exposed toward the extracellular environment. In this way, by changing the amount of receptors in the plasma membrane, endocytosis may modulate the response of the cell to external signals. Some pathogens, such as bacteria and viruses, use the endocytosis pathway to enter and infect the cells.
1. Selection of molecules
Molecules can be endocytosed non-specifically by pinocytosis, specifically by receptor mediated endocytosis, and as part of the membrane of the endocytic vesicle or compartment (Figure 1).
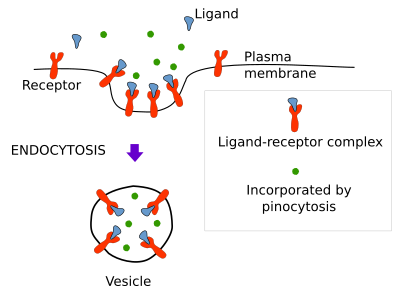
Pinocytosis
Pinocytosis is a non-specifically way for soluble molecules to get into the endocytic compartments (Figure 1). No doubt that each endocytic vesicle contains soluble molecules that came in randomly. Hence, all endocytic pathways carry out pinocytosis. However, macropinocytosis is specialized in this type of non-specific endocytosis (see below).
Receptor mediated endocytosis
The receptor mediated endocytosis is a mechanism for capturing specific extracellular molecules recognized and bound by plasma membrane receptors (Figure 1). More than 25 types of receptors have been found to participate in this type of endocytosis. They allow the cell to efficiently fetch molecules and particles very diluted in the extracellular matrix. The molecule (ligand)-receptor complexes converge in a spot of the plasma membrane where the endocytic vesicle is going to be formed. Cholesterol enters the cell by receptor mediated endocytosis. It is transported in the blood as part of the low density lipoproteins (LDL). LDL are large molecular complexes containing many molecules of cholesterol surrounded by a lipidic layer and a protein. When a cell needs cholesterol, it synthesizes many receptors for LDL that are transported to the plasma membrane. In the cell surface, the receptors recognize and bind LDL complexes, they gathered in a region where an endocytic vesicle is formed. The vesicle pinches off and is targeted to inner organelles where LDL releases cholesterol, which is then metabolized. The inhibition of this endocytic pathway for capturing cholesterol, mostly by failures of the LDL-receptor recognition, or because there are no synthesis of receptors, may lead to cholesterol to accumulate in interstitial spaces and cause arteriosclerosis and heart attack.
2. Types of endocytosis
A ttending to the size and type of vesicle or compartment, how it is formed, and the type of material to be endocytosed, several types of endocytosis have been described. We will deal with clathrin coated vesicles, caveolae, non-coated vesicles, and macropinocytosis. We also address phagocytosis, a special type of endocytosis for capturing large particles, such as bacteria and cellular leftovers (Figure 2).
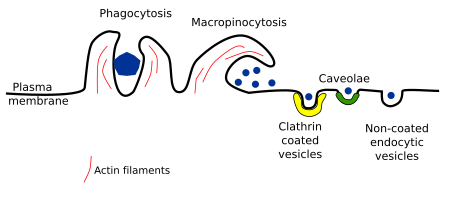
Clathrin coated vesicles
The endocytosis mediated by clathrin coated vesicles is the main pathway to internalize plasma membrane integral proteins and lipids, as well as extracellular molecules and molecular complexes that do not usually exceed 156 nm in size, including some viruses. This endocytic mechanism is constitutive and is always working. It has been suggested as a housekeeping mechanism. Clathrin coated vesicles are formed in plasma membrane regions where clathrin proteins are recruited from the cytosol. In fibroblasts, these regions account for about 2 % of the total surface of the plasma membrane. The clathrin is a three arms protein that allow the auto-assembling of many proteins to form pentagons, which help to form vesicles of about 120 nm. Between the clathrin coat and the plasma membrane, there are proteins involved in many aspects of the vesicle formation. For instance, adaptor proteins select the cargoes. Once the vesicle is formed, it detaches and moved away from the plasma membrane. Then, the clathrin coat is disassembled and the "naked" vesicle is targeted to inner compartments, mostly early endosomes
Clathrin coated vesicles are formed in 20 to 120 seconds, limited by cargo selection and recruitment of cytosolic clathrin proteins. Actually, the majority of initiated clathrin coated vesicles do not complete the process. A clathrin coated vesicle is formed every 2 minutes per µm2 of plasma membrane in macrophages and epithelial cells.
Caveole
Caveolae were observed in the 1950s by P. Palade by using transmission electron microscopy. They are small invaginations (45-80 nm) of the plasma membrane found in most eukaryote cells. It is supposed that many caveolae become vesicles, but it is not clear the proportion. Caveolae are abundant in endothelial cells, muscle cells and adipocytes. In some cells, such as muscle cells, caveolae may be up to 50 % of the plasma membrane, whereas they are scarce in hepatocytes, or even absent in the cells of the proximal convoluted ducts of the kidney. Several caveolae may associate and establish connections between one another by membrane necks, forming the so called caveolar rosettes. Caveolae have been observed in the Golgi apparatus, so that they may work as a shuttle vesicular mechanism communicating the Golgi apparatus with the plasma membrane. Most vesicles coming from caveolae fuse with early endosomes. Some authors suggest that they are a particular type of early endosomes referred to as caveosomes.
The membrane of caveola contains a particular lipid and protein composition. It contains a high proportion of cholesterol and many sphingolipids (sphingomyelin and glycosphyngolipids). There are two typical proteins: caveolin and cavin, as well as glycosyl phosphate inositol-anchored proteins, Caveola membrane proteins can be classified as central and accessory. Caveolin 1 and 3, cavin 1 and pacsin/sindapin are central proteins. Caveolin is an essential protein because it is enough to form caveolae. For instance, the artificial expression of caveolin in bacteria leads to the formation of caveolae, so that it is enough to form membrane invaginations. In eukaryote cells, caveola formation needs caveolin and cavin1. Actually, oligomerization of caveolin may lead to a particular lipidic environment in the membrane. There are 100 to 200 caveolins per caveole, and different types of caveolins can be found in one caveole. In mammals, there area caveolin 1, 2 and 3. Caveolin 1 is expressed in most cells, and it is necessary to form caveolae. Caveolin 2 is expressed with caveolin 1, but it is not essential for forming caveolae. Caveolin 3 is expressed in skeletal muscle, cardiomiocytes and some other non-muscle cells. It is needed for caveolae formation in these cells. Caveolins are small proteins that recruit cholesterol. Both, carboxyl and amino terminals of caveolins are cytosolic, and the protein is inserted in the membrane as a wedge, so that they fold the membrane. These proteins are synthesized in the endoplasmic reticulum and accumulate in the Golgi apparatus, where they associate and form oligomers. Cholesterol is necessary for the maturation of these oligomers and for exporting them toward the plasma membrane. Caveolins remain together in the plasma membrane, where they begin to recruit other molecules, lipids and proteins, to form the caveole.
Las cavins are recruited by caveolins and associated to the membrane cytosolic surface of caveolae. There are about 50 cavins per caveole. They can bind lipids and stabilize the formation of caveole. Other proteins are also found in caveolae, such as EHD proteins (Eps 15 Homology Domain), which are related to the neck of the invagination, and pacsins with an F-BAR domain for bending the membrane. Pacsins may also be involved in recruiting dynamin and EHD proteins. In vertebrates, caveolins, cavins and pacsins are necessary to get a normal caveole.
The split of the caveole from the plasma membrane is driven by dynamin proteins. The new vesicle may fuse again with the plasma membrane or with an early endosome. It is interesting that the membrane of the caveole vesicle remains as a patch in the endosome membrane, and may invaginate again to form a new vesicle that is moved to the plasma membrane. This movement is ruled by microtubules and actin filaments.
The functions of caveolae are not fully known yet. Although, they have been suggested to perform a main role in endocytosis, it has not strong experimental support. Besides endothelial cells, muscle cells and adipocytes, it is estimated that only 5 % of the caveolae ends up as vesicles, at least in cell cultures. Only 1 % of caveolae becomes vesicles in one minute in fibroblasts and adipocytes. Therefore, other functions have been posed. Caveolae may modulate the cellular signaling by removing receptors from the cell surface, such as tyrosine kinase receptors. They also can influence the lipid trafficking between the plasma membrane and the inner organelles. They have even been suggested to be involved in the development of some tumors. Choleric toxin, folic acid and other molecules enter the cell by caveolae. The number of caveolae may also influence the intensity of other types of endocytosis, such as clatrhin mediated endocytosis, because caveolae may seize proteins and lipids, therefore changing the plasma membrane composition. Another currently explored function of caveolae is the ability to modify the mechanical tension of membranes, son that the number of caveolae affects the physical properties of the plasma membrane. This may explain why caveolae are so abundant in endothelial cells, muscle cells and adipocytes, cells under a heavy mechanical stress. In endothelial cells, caveolae also participates in transcellular transport or transcytosis.
Non coated vesicles
The endocytic vesicles that do not need clathrin or caveolins are called non-coated vesicles. This endocytosis pathway was uncovered because endocytic vesicles were formed when both clathrin coated vesicles and caveolae were specifically inhibited. The non-coated vesicle formation seams to need a signal to be activated, but it is not known the molecular mechanisms for selecting cargoes. It was suggested that lipid rafts may play a role in this endocytic pathway.
Sometimes, cells need to produce son many vesicles so quick that clathrin is no enough to fulfill the task. For instance, there are hundreds of vesicles in the synaptic terminals ready to get fussed with the membrane when the action potential arrives. However, when many action potentials are incoming to the synaptic terminal in a short period of time, the vesicle pool gets exhausted, and the terminal is not able to respond to a new incoming action potential. New vesicles have to be formed in these periods of high activity from the plasma membrane by endocytosis, probably by non-coated vesicles. Clathrin coated vesicles only supply enough vesicles under regular neuronal activity. In addition, after a massive fusion of vesicles with the membrane of the synaptic terminal, an excess of membrane needs to be removed to keep constant the amount of the terminal membrane. This mechanism has been found in other cell types, such as epithelial cells.
There are several types of very fast endocytosis: macropinocytosis (see below), massive activity-dependent endocytosis, endophilin-mediated endocytosis, "kiss and run" endocytosis, and ultrafast endocytosis. All of them need a signal to be activated that can be sensed by receptors or by other mechanisms. Endophilin is a protein participating in the non-coated vesicle formation. These vesicles can be formed in 1 to 10 seconds, so that it is called FEME (Fast Endophilin Mediated Endocytosis). It is not a constitutive pathway. Cholera and shiga toxins can enter the cell through this type of endocytosis. The process begins when endophilin binds to activated receptors (G protein associated receptors, interleucine-2 receptors, and tyrosine kinase receptors), which quickly recruit synaptotagmin and dynamin for boosting the vesicle formation. Endophilin contains BAR domains for bending the membrane.
Macropinocytosis
Large amounts of extracellular material are endocytosed by macropinocytosis. It begins with protrusions at the cell surface, as waves in the sea, with a rather circular base. The crests of the cell expansions fuse to one another, or fall onto the cell surface, to form a large compartment of about 0.2 to 10 µm referred to as macropinosome. Actin filaments and myosin motor proteins are involved in macropinocytosis. The material internalized within the micropinosome is nonspecifally captured. The process of protrusion formation begins with the polymerization of a large ring, or crescent, of actin filaments under the plasma membrane with several µm in diameter. From this ring, actin filaments polymerize perpendicularly to the cell surface to form the protrusions. The place where the ring is assembled is enriched in Ras proteins and PIP3 lipids. PIP3 may recruit the proteins that drive active polymerization and plasma membrane expansion, such as myosin. Once the macropinosome is formed, PIP3 disappears from its membrane.
It is suggested that macropinocytosis is an ancient cellular mechanism to fetch food. In fact, there is a molecular connection between macropinocytosis and lysosomes through mTOR, a protein degradation sensor. However, macropinocytosis develops other roles in metazoans. Thus, it can be induced by cytokines, chemokines, pathogens and cellular activity. Some cell types look like having a constitutive macropinocytosis. For instance, macrophages increase the cleaning capacity by macropinocytosis, and dendritic cells may use macropinocytosis to capture large amount of extracellular material and present antigens to T lymphocytes after the processing of the internalized material. However, macropinocytosis is commonly an induced process. Neurons may perform macropinocytosis to recover membrane after a period of heavy exocytosis. Cancer cells activate macropinocytosis to feed on the extracellular material. Many pathogens enter the cells by micropinocytosis, such as bacteria, the ebola virus, and even prions.
Phagocytosis
Phagocytosis is a particular type of endocytosis where large particles are internalized, such as bacteria, cell debris and viruses. Macrophages, dendritic cells and neutrophils are specialized cells doing phagocytosis. For instance, macrophages capture particles (virus, bacteria, cells) with immunoglobulins attached. They also remove thousands of erythrocytes every day. Macrophages are resident in tissues, like Kupffer cells in the liver, microglia in the encephalon, and others in the bone marrow to remove the erythrocyte nuclei in mammals during red blood maturation. Protozoans feed by phagocytosis. It is plausible that phagocytosis evolved from a feeding mechanism to a defense and cleaning process in pluricellular organisms.
The first step to initiates phagocytosis is to recognize a molecule in the particle to be captured. Then, protrusions (pseudopods) are extended from the cell surface to engulf the particle. The recognized molecules may be molecules at the surface of pathogens, phosphatidylserine in apoptotic cells, or immunoglobulins/complement complexes of the immune system. If there are enough amounts of these molecules, phagocytosis is triggered. However, if the number of molecules to be recognized is small, phagocytosis does not start. Macrophages harbor a variety of receptors that cooperate to recognize a particle and increase in this way the intensity of the recognition.
Actin filaments and myosin proteins assemble the cell protrusions. These extensions surround, fuse their edges and enclose the particle, finally forming a really large compartment, called phagosome, within the cell. Phagosoma is moved to inner regions of the cytoplasm and gets fused with lysosomes for particle degradation.
-
Bibliography ↷
-
Bibliography
Bloomfield G, Kay RR. 2016. Uses and abuses of macropinocytosis. Journal of cell science. 129: 2697-2705.
Cheng JPX, Nichols BJ. 2016. Caveolae: one function or many? Trends in cell biology. doi: 10.1016/j.tcb.2015.10.010.
Mayor S. Pagano RE. 2007. Pathways of clathrin-independent endocytosis. Nature reviews in molecular and cell biology. 8:603-612.
Watanabe S, Boucrot E. 2017. Fast and ultrafast endocytosis. Current opinion in cell biology 47:74-71.
-
 Exocytosis
Exocytosis 