1. Morphology
2. Grow and Proliferation
3. Functions
- Energy storage
- Endocrine
- Protection
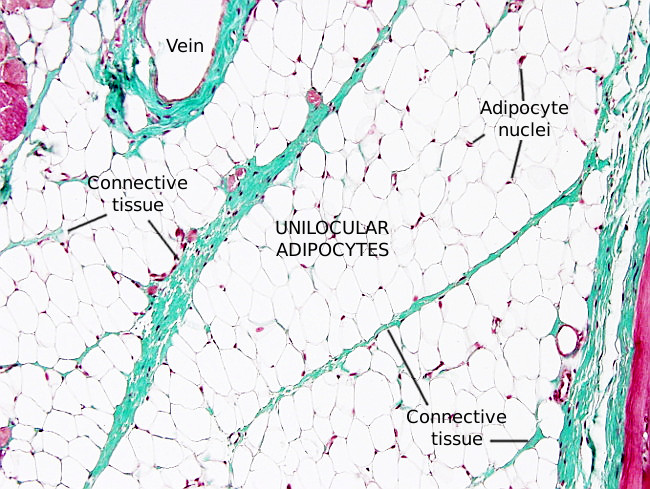
Unilocular adipocytes, or white adipocytes, are found in the adipose tissue, although they are also observed scattered through the connective tissue. The white color, sometimes yellowish, is the color of the fresh white fat. Adipocytes are specialized cells in storing energy as neutral fatty acids when the energy balance is positive. Unilocular means that the majority of the cell cytoplasm is occupied by a very large lipid droplet. Most adipocytes of the body are white adipocytes. There are other types of adipocytes such as multilocular or brown adipocytes, beige adipocytes and pink adipocytes. In mice, adipocytes are classified as white, brown and beige, depending on the morphology.
1. Morphology
Unilocular adipocytes are usually rounded in shape. The cell size is variable, and they can be up to 100 to 150 µm in diameter when filled with lipids (Figures 1 and 2). Adipocyte diameter changes during development. In humans, it is about 40 to 50 µm in the fetus, 50 to 80 µm in newborns, 90 to 130 µm in babies, 50 to 200 µm in normal adults, and 90 to 270 µm in obese adults. The maximum size is limited by the oxygen diffusion and by the interactions with the extracellular matrix.
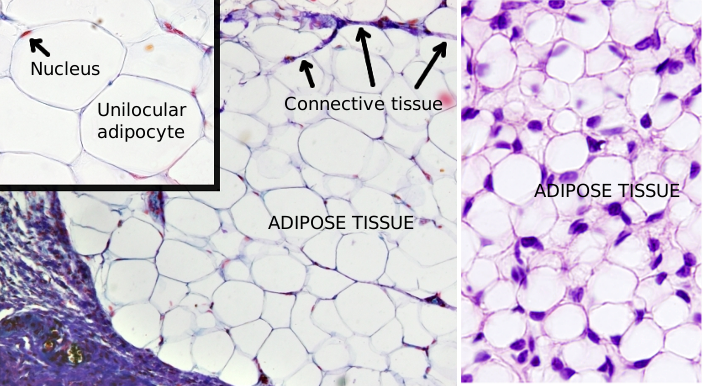
Unilocular adipocytes contain a large lipid droplet that occupies most of the cytoplasm. Lipid droplets are found in all eukaryote cells, which means they perform an essential function for the cell. They are not limited by a bi-layered membrane, but by a lipid membrane monolayer of about 5 nm in thickness, which is derived from the endoplasmic reticulum membrane. This monolayer is covered by a network of vimentin filaments. Lipid droplets may be in contact with endoplasmic reticulum and mitochondria, facilitating the esterification and degradation of triacylglycerol, respectively. Some cisterns of endoplasmic reticulum have sometimes been observed inside the lipid droplets, indicating the source of these lipid organelles. The remaining organelles and the nucleus are squeezed against the plasma membrane. Unilocular adipocytes contain the same set of organelles than other cells have, like endoplasmic reticulum, Golgi apparatus, and mitochondria. During the formation of the lipid droplet, there is an intense endocytic flux mediated by caveolae, which can be observed at transmission electron microscopy. Caveolae may be up to 30 % of the total membrane of the adipocyte.
Unilocular adipocytes are usually found forming irregular hexagonal groups. In each group, there are cell-cell connections by gap junctions that allow adipocytes to respond to electrical inputs in a coordinated manner. It is unknown if these cytoplasm-cytoplasm connections also happens between adipocytes of different groups.
Lining the external surface of the plasma membrane of mature adipocytes, there is a layer of extracellular matrix known as external lamina, with similar features as the basal lamina of epithelia. The external lamina may function as a selective barrier or as a mechanical scaffold, or both. It contains type IV collagen, laminin and heparan sulfate, but lacks fibronectin. Fibronectin is present, however, in non-mature adipocytes, and it is later exchanged for laminin.
The extracellular matrix is very important for unilocular adipocytes because it influences cell size and differentiation. For instance, it has been suggested that abundant type IV collagen is involved in adipocyte cellular size regulation. In addition, pre-adipocytes are not differentiated into mature adipocytes until they are able to release MT1-MMP metalloproteases. Metalloproteases are enzymes that degrade extracellular matrix. Thus, extracellular matrix may physically regulate the adipocyte hipertrophic growth. Adipocytes are connected to the extracellular matrix through integrins, which can bind laminin, fibronectin and collagen. There are changes in the expression of integrin types that guide adipocytes through the cell differentiation process. Integrins may also be a cell-size sensor.
2. Grow and proliferation
During the human embryo development, visible at 6 months of pregnancy, there are cells with small lipid droplets in the hypodermis and among viscera. These cells increase in size from 15 µm to 80 µm during birth time.
Ahite adipose tissue can grow by increasing the size of preexisting adipocytes (hypertrophy) and by adipocyte proliferation (hyperplasia or adipogenesis). Both mechanisms may happen when the net body energy is positive.
The increase of adipocyte size is the first step to obesity. If energy is still coming, the adipose tissue response is the proliferation of adipocytes. Adipocyte hyperplasia happens during severe obesity. Adipocytes can grow to a maximum size and, when this limit is reached, proliferation of undifferentiated pre-adipocytes begins. Large size adipocytes release paracrine substances that stimulate pre-adipocytes to proliferate. Thus, hypertrophy usually precedes adipogenesis during white fat growing. Large adipocytes become resistant to insulin. In humans, adipocyte hypertrophy are related to a higher risk of having type II diabetes, regardless of the general obesity. Furthermore, the inhibition of hyperplasia may lead to ectopic white fat depots in the liver and muscles, which can produce obesity related diseases.
Adipogenesis is produced by a population of fibroblast-like mesenchymal stem cells found in the adipose tissue, a population that is active along the individual life. Mesenchymal cells are derived from the neural crests (ectoderm) and mesoderm (excepting the intermediate mesodermo). There are two steps during adipocyte differentiation: commitment, when mesenchymal cell becomes pre-adipocyte, and differentiation, when pre-adipocyte becomes mature adipocyte. These stem cells are found around the blood vessels. However, during early stages of development, the number of adipocytes is genetically determined.
Proliferation of adipocytes in different body fat depots depends on several factors, such us the precursor adipocyte population present in the depot, blood perfusion and density of nerve innervation. For example, there is an inverse relation between the density of nerves and the increase in adipocyte proliferation. Likewise, some hormones like insulin and testosterone influence adipocytes behavior, but appear to have more impact on the adipocyte size than in proliferation. There are two main white fat depots in the body: subcutaneous and visceral. Hyperplasia is more frequent in the subcutaneous depot and hypertrophy is prevailing in the visceral depot. The origin of these two depots is different, and their physiology and function are also different.
3. Functions
The main function of adipocytes is to store energy in lipid molecules. However, they are also involved in regulating levels of glucose and lipids in the organism. Furthermore, the functions of adipocytes are different depending on the white fat depot.
Energy storage
Adipocytes storage energy as neutral triacylglycerols, so that they can be released during exercise or in periods where food is scarce. From the extracellular space, adipocytes can uptake fatty acids, cholesterol and glucose. Glucose is transformed in fatty acids. Adipocytes are very flexible when storing / releasing energy. They store fatty acids during the periods with abundance of food, which are released when the body needs energy. Lipids are stored more easily than glycogen, because lipids are not soluble molecules. Furthermore, a quantity of lipids can contain double amount of energy that the same quantity of glucose. One gram of lipids stores 38 KJ. The lipid storing process is not just to store energy, but also for clearing circulating fat and avoiding ectopic lipid depots. An excess of lipids is toxic (lipotoxicity), particularly dangerous for muscle and liver.
About 80 % of free cholesterol (non-esterified cholesterol) is found in the membrane of lipid droplets. Fat depots work as a sink of cholesterol in normal adult humans. Around 25 % of the total cholesterol of the human body is found in fat depots. Caveolae are abundant in adipocytes, which contain the same amount of protein caveolin 1 than caveolin 2. The interactions of the two caveolins is important for caveolae formation in adipocytes, which is mediated by cholesterol. Insulin receptor is found in caveolae membranes. During lipid droplet growing, caveolins are moved from the plasma membrane to the lipid droplet membrane, concurrently with the uptake of large amounts of cholesterol. In fact, it is cholesterol that activates the caveolins transfer to facilitate the lipid droplet expansion.
Lipogenesis is the process for uptake and store triacylglycerols in adipocytes (Figure 3). The major pathway for synthesizing lipids begins with glycerol 3-phosphate, which is transformed into mono-, di-, or triacylglycerols by fatty acid esterification. This process happens in the endoplasmic reticulum. The hormone insulin favors this process by increasing the uptake of glucose by adipocytes. Glycerol 3-phosphate is synthesized from glucose, although it can also be produced from other sources like lactate and some amino acids. Insulin has an additional role by inhibiting lipolysis. Glucose crosses the adipocyte plasma membrane through the GLU4 glucose transporter. This receptor is removed from the plasma membrane by endocytic vesicles and stored in endosomes when insulin levels are low. When insulin concentration increases again, GLU4 is moved to the plasma membrane again and uptakes glucose.
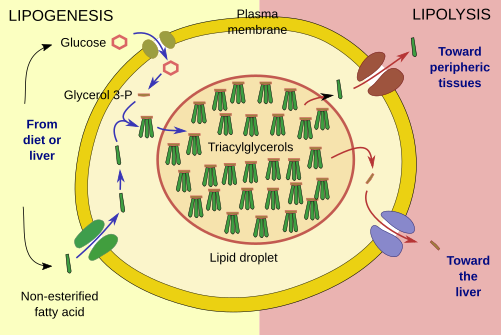
Lipolysis is the degradation of lipid droplet triacylglycerols into fatty acids (Figure 3). The enzymes involved in lipolysis are found associated to the surface of the lipid droplet. After a hormonal stimulus, they are massively recruited from the cytosol. The fatty acids are released to the extracellular space.
Endocrine
Besides the energy storing function, unilocular adipocytes are very active cells releasing proteins and hormones, altogether known as adipocines. Adipose tissue is the largest endocrine structure of the body and can release more than 500 active molecules. Some of them have inflammatory activity. Leptin and adiponectin are adipocines involved in metabolism. Leptin regulates food intake by acting on the hypothalamus. Adiponectin induces sensibility to insulin in the liver and muscles. Adipocytes also release molecules that modulate the immune system, blood vessel formation and remodeling of the extracellular matrix.
Protection
The subcutaneous depot of white fat works as a temperature insulation tissue to protect against lower temperatures. Furthermore, subcutaneous fat depots absorb mechanical strokes on the skin of animals.
-
Bibliography ↷
-
Haczeyni H, Bell-Anderson KS, Farrell GC. 2018. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obesity review. 19: 406-420.
Hausman DB, DiGirolamo M. Bartness TJ. Hausman G J, Martin RJ. 2001. The biology of white adipocyte proliferation. Obesity review. 2: 239-254.
Pope BD, Warren CR, Parker KK, Cowan CA. 2016. Microenvironmental Control of Adipocyte Fate and Function. Trends in cell biology. 26:745-5
Rutkowski JM, Stern JH, Scherer PE. 2015. The cell biology of fat expansion. Journal of cell biology. 208: 501-512.
White U, Ravussin E. 2019. Dynamics of adipose tissue turnover in human metabolic health and disease. 62: 17–23.
-