1. Organization
2. Distribution
3. Early
4. Multivesicular
5. Acid hydrolases
6. Transcytosis
7. Exosomes
8. Macroautophagy
9. Pathology
Endosomes are membrane-bound compartments showing irregular shapes, like large "bags", although sometimes they organize tubular structures. They are stations for receiving and shipping molecules packed in vesicles. Endosomes are essential for cell homeostasis. Vesicles arrive to endosomes from plasma membrane through the endocytic pathway, and from the TGN of the Golgi apparatus. Intracellular compartments formed after phagocytosis and macropinocytosis become particular types of endosomes known as phagosome and macropinosome, respectively. Most molecules of the endosomes are targeted to lysosomes for degradation. However, there are also vesicles leaving the endosomes toward the plasma membrane or TGN of the Golgi apparatus, both are recycling pathways for membrane receptors and lipids. Endosomes also exchange molecules with other compartments, mostly the endoplasmic reticulum, by membrane contact sites.
1. Organization
A cell may contain a diverse set of endosomes, both morphologically and functionally. An endosome may even show domains in its membrrane with different roles. The different types of endosomes are actually a stages of a continuous maturation process, where a type of endosome becomes another type. The whole endosome population of a cell is called endosomal compartment. The main stages of endosomal maturation are early endosomes, recycling endosomes, sorting endosomes, and multivesicular/late endosomes. However, there are two main types: early (earle, sorting and recycling) and late (multivesiscular, late) endosomes. Some authors include the lysosomes into the endosomal compartment. We deal with lysosomes in the next page.
Aach endosome performs a particular functions in the cell. Early endosomes are arrival stations for endocytosis vesicles, the sorting/recycling endosomes ship vesicles toward the plasma membrane and trans Golgi network domain, the multivesicular/late endosomes are bidirectionally connected with Golgi apparatus by vesicles. The multivesicular bodies/late endosomes finally fuse with lysosomes for molecular degradation.
The identity of each type of endosome relies on the molecular repertory of its membrane, mainly by the type of Rab and Arf/Arl GTPases, the interactions with effector proteins, and the lipid composition of the membrane. A progressive maturation of the endosomes happens by changing the molecular composition. Early endosomes are formed by fusion of endocytosis vesicles. They contain Rab4, Rab5, and are enriched in the phosphoinositide PI(3)P. The early endosomes move toward the inner regions of the cytoplasm and becoming sorting and recycling endosomes, releasing vesicles that go fuse back with the plasma membrane. At this stage Rab4 is sunsituted byu Rab7. Recycling/sorting endosomes become multivesicular/late endosomes, that harbor Rab7 and the phosphoinositide PI(3,5)P2. Finally, multivesicular/late endosomes fuse with lysosomes. In this way, each type of endosome is a stage in the maturation pathway, that it is altogether known as endosomal maturation.
2. Distribution
Different endosomal types are distributed in particular cytoplasm regions (Figure 1). Early endosomes are found near the plasma membrane, the cytoplasm periphery. As they mature, they move to the inner cytoplasm as recycling/sorting endosomes, and are finally concentrated at the perinuclear region as multivesicular bodies/late endosomes that finally fuse with lysosomes. It is suggested that the peripheral endosomal system is more related to cell signaling, and the perinuclear one more with material degradation. The set of perinuclear endosomes is known as endosomal cloud. In this way, the material is acquired in the cell periphery, recycled during the journey, and degraded in deeper places of the cytoplasm. Arf, Rab and Arf-like (Arl) proteins are involved in establishing the position and movement of endosomes within the cell. It is of notice that late endosomes maintain the perinuclear position by interacting with the endoplasmic reticulum. Actually, endoplasmic reticulum may also be involved in fission and fusion of endosomes, similarly as it does with mitochondria. Anyway, long distance movements of endosomes are driven by microtubules, and short movements by actin filaments.
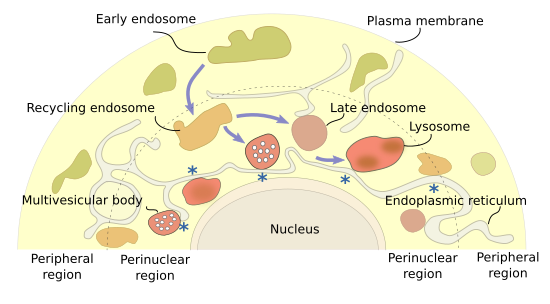
3. Early endosomes
Early endosomes are the target of endocytic vesicles (Figure 2). They are named recycling/sorting endosomes when they are able to send vesicles back to the plasma membrane. The recycling flux can be very intense and returns to the plasma membrane up to 90 % of the proteins and 60 % of the lipids previously endocytosed. Early/recycling endosomes have an acid pH (around 6.5) compared to the cytosolic pH (around 7.2). This difference is due to ATP-dependent proton pumps found in the endosomal membrane. The slightly acid pH inside the endosomes leads to the release of ligands from their receptors. The receptor-ligand complexes were previously included in endocytic vesicles. In this way, receptors may be sent back to the plasma membrane by recycling vesicles, while ligands can follow the degradation pathway toward lysosomes. From recycling/sorting endosomes, vesicles are also targeted to the trans domain of the Golgi apparatus.
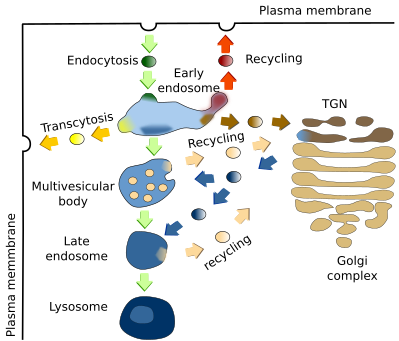
The vesicles are released by early endosomes at particular endosomal membrane domains. It is actually suggested that there are several domains in the endosomal membrane: a domain for docking and fusion of endocytic vesicles, another for shipping vesicles toward the plasma membrane, a domain which is going to be transformed into multivesicular bodies/late endosomes, and another one to send vesicles to the Golgi apparatus. Some authors pose other less known domains.
4. Multivesicular bodies / late endosomes
Multivesicular bodies/late endosomes (Figure 2), are the previous step for degrading endocytosed molecules, which finally occurs in lysosomes by the activity of acid hydrolases. Molecules for degradation are coming from early endosomes, and acid hydrolases are packaged in vesicles in the TGN of the Golgi apparatus targeted to multivesicular bodies / late endosomes. Recycling vesicles carrying receptors and membrane are released from multivesicular bodies / late endosomes back to the TGN of the Golgi apparatus. The formation of the recycling vesicles are mediated by a group of proteins called retromeres, which select the cargoes and help with the formation of the vesicles.
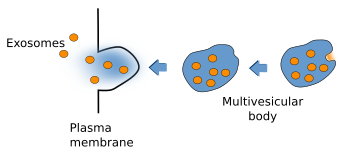
Multivesicular bodies were first described according to their morphological features (Figure 3). They are rounded organelles with a membrane enclosing a variable number of inner vesicles (from two to dozens). Typically, multivesicular bodies are 250 to 1000 nm in diameter. They can occasionally show some tubular structures, and are moved through the cytoplasm by means of microtubules. The inner vesicles (30 to 60 nm in diameter) are formed after small invaginations of the multivesicular body membrane. In this way, it is possible to degrade membrane proteins and lipids, and also small cytosolic portions incorporated inside each vesicle. The multivesicular body content may be degraded in lysosomes or released into the extracellular space by fusion with the plasma membrane. The fusion of multivesicular bodies with the plasma membrane releases the inner vesicles to the intercellular space, and the vesicles are then renamed as exosomes. There is still another possibility in the synapses of the nerve tissue, where vesicles from multivesicular bodies may fuse with the plasma membrane. This mechanism is referred to as back-fusion. It may explain why this organelle is frequently observed in synaptic terminals, but it is rare in the post-synaptic elements.
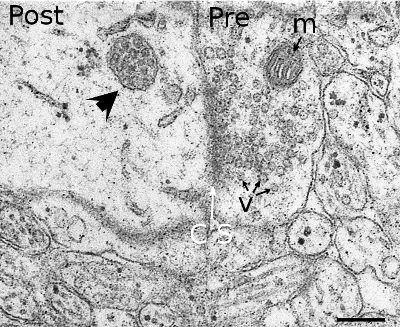
The material of multivesicular bodies/late endosomes is usually targeted for degradation in lysosomes. Two non-exclusive ways may happen: maturation of endosomes into lysosomes or fusion of endosomes with lysosomes.
5. Acid hydrolases
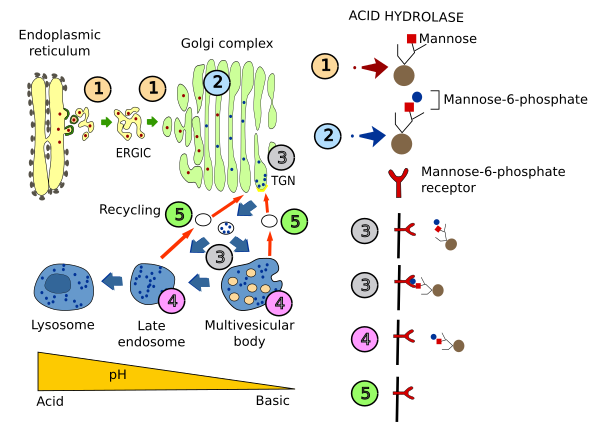
Acid hydrolases are synthesized and included in vesicles in the endoplasmic reticulum. After being transiently part of the cis domain of the Golgi apparatus, these enzymes are then moved to the trans Golgi network (TGN). In the cis domain, a phosphate group is added to one mannose (mannose-6-phosphate) of the hydrolases. Acid hydrolases are then segregated from other TGN molecules by a transmembrane receptor that recognizes their mannose-6-phosphate moiety. Receptor-hydrolase complexes are then gathered at membrane areas enriched in clathrin, where vesicles enclosing the receptor-hydrolase complexes are formed. These vesicles are targeted to multivesicular bodies/late endosomes, and fused with them. The acidic pH inside these organelles, compared with that of the TGN, makes possible the release of hydrolases from their receptors. In addition, the phosphate group of the hydrolases is removed. The now free receptors are packaged again in vesicles going back to the Golgi apparatus. Meanwhile, hydrolases continue their way toward lysosomes.
5. Transcytosis
In some cell types, endosomes are involved in another vesicular pathway known as transcytosis. When ligand-receptor complexes arrive at early endosomes by endocytosis, ligands are not released but ligand-receptor complexes are included in another vesicle and targeted to another plasma membrane domain different from that where endocytosis happened, and there it releases its content, that is, the ligand. This vesicular pathway happens in polarized cells, such as epithelial cells. For instance, by this mechanism iron is transferred from food through the intestine epithelium into the interstitial milieu.
6. Exosomes
vesicles
Some cell types (hematopoietic cells, dendritic cells, intestine epithelial cells, mastocytes and tumor cells) show some peculiarities in their vesicular trafficking. Besides becoming lysosomes, or fusing with them, multivesicular bodies are able to fuse to the plasma membrane and release their inner vesicles to the extracellular space (Figure 5). These released vesicles are known as exosomes. They contain a distinct molecular composition compared with other cell compartments. For example, cholesterol and sphingomyelin are abundant in their membranes. Exosomes have been proposed as message carriers between cells.
7. Macroautophagy
Endosomes may be formed by a mechanism different from endocytosis. Eukaryote cells are able to digest their own content by a mechanism known as autophagy. It is a basal constitutive mechanism that can be started or boosted by cellular stress, like food deprivation, or by cell signalling. Macroautophagy is a type of autophagy consisting in enclosing cytosolic material, including soluble material and organelles, with membranes, probably coming from the endoplasmic reticulum. It results in a large membrane bound compartment known as autophagosome that fuses with lysosomes. This last behavior, the fusion with lysosomes, is similarly performed by multivesicular bodies/late endosomes. So, it is suggested that autophagosome is kind of endosome.
8. Pathology
Many types of bacteria hack the endocytic pathway, mostly through phagocytosis and macropinocytosis, to enter the cell. The internal acid environment of endosomes is favorable for bacteria to first proliferate and then cross the endosomal membrane. Some species of bacteria house inhibitory molecules to prevent the fusion of endosomes with lysosomes, and escape from be degraded. Many types of virus also use endosomes to infect cells.
-
Bibliography ↷
-
Bibliography
Bibliografía
Cabrera M, Ungermann C. 2010. Guiding endosomal maturation. Cell. 141:404-406.
Jongsma MLM, Bakker N, Neefjes J. 2023. Choreographing the motor-driven endosomal dance. Journal of cell sciences. 136, jcs259689. doi:10.1242/jcs.259689.
Neefjes J, Jongsma MML, Berlin I. 2017. Stop or go? Endosome positioning in the establishment of compartment architecture, dynamics, and function. Trends in cell biology. 27:580-594.
Rind HB, Butowt R, von Bartheld CS. 2005. Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin. Journal of Neurosciences. 25, 539-549.
-
 Endocytosis
Endocytosis 