Vascular plants, unlike non-vascular plants, have specialized tissues for transporting water along with both inorganic and organic dissolved substances. These conducting tissues are known as vascular tissues, which include the xylem and phloem. Vascular tissues emerged around 450 million years ago, when plants began to colonize the land. During evolution, lycophytes were the first to show vascular tissues. These tissues allowed plants to connect the two main domains of the land plants: the aerial part, which performs photosynthesis to make organic molecules, and the underground parts, which absorb water and minerals. The xylem specializes in transporting water, inorganic compounds, and some organic substances from roots to the rest of the plant organs, while the phloem mostly carries organic substances synthesized in plant body organs, primarily in leaves and storage tissues, toward the rest of the plant. More than 300,000 species of vascular plants are currently known.
1. Introduction
Vascular tissues perform three main functions in plants. a) Physiologically, plants need vascular tissues to increase their size by distributing water and organic substances to feed the cells. Although nearly every plant cell can exchange substances with the adjoining cells through plasmodesmata, which are openings that directly communicate two cytoplasms across the shared cell wall of neighboring cells, this is a short-distance transport that only reaches a few cells away. Thus, for long-distance transport, a specialized tissue is needed: the vascular tissue. b) Vascular tissues also play a mechanical role by supporting the aerial parts and giving consistency to the underground organs, acting as a skeleton. c) Another function of vascular tissues is to allow communication between distant organs of the plant body by transporting signalling molecules, including phytohormones, ARNs, proteins, and others.
Vascular tissues arise during embryonic development, before germination. During this period, provascular cells differentiate (4 provascular cells in A. thaliana) between the epidermis and the ground tissue and are situated in the interior of the embryo. Provascular cells proliferate and become the procambium meristem within the caulinar and root apical meristems, which gives rise to the primary vascular tissue. The procambium maintains the production of primary xylem and phloem in the inner regions of the plant after the apical meristems are far away due to organ growth. The formation of primary xylem and phloem happens after germination. Certain cells of the apical meristem may also differentiate directly into vascular cells. The primary vascular tissues are protoxylem and protophloem, which are subsequently replaced by metaxylem and metaphloem as the main functional conducting tissues during maturation of organs. Metaxylem and metaphloem also arise from the procambium meristem, and are primary vascular tissues too. When secondary growth takes place in the plant, the secondary xylem and secondary phloem are formed from the vascular cambium meristem, while the metaphloem and metaxylem become nonfunctional. At the same time, the caulinar and apical meristems continue to produce procambium (primary vascular tissues) as the stem and roots grow in length.
There is a longitudinal and a transversal arrangement of the vascular tissues, although it varies with the type of organ, plant age, and plant species. The basic organization of the vascular tissues is under the control of auxin and cytokinin hormones. Several types of cells make up the vascular tissues, some of which have been key elements for evolutionary studies. Most of these cell types derive from the same meristematic cell, which is why xylem and phloem are physically close.
Stele
The set of vascular tissues of a shoot or root is known as the stele. The type of stele is determined by the plant species, branching pattern, leaf arrangement, gradient and concentration of auxin, and everything under the influence of the genome. The auxin released from leaf primordia at the apex of the stem determines the disposition of new leaves and vascular bundles, as the auxin influences the differentiation of primary vascular tissues.
The stele is named according to the organization of the vascular tissues (Figure 1). When the vascular tissues form a cylinder without medullary parenchyma, the stele is named as the protoestele. If the core of the cylinder is medullary parenchyma, the stele is known as the siphonostele. A eustele is a type of siphonostele with the wall of the cylinder showing discontinuities, and it is formed of many vascular bundles. The eustele is typical of dicots with primary growth and monocots at their stem apical regions. In monocots, the eustele is transformed into an atactostele by branching the vascular bundles. The atactostele is typical of monocots. If we attend to the location of xylem and phloem, there are various types of organizations (Figure 1). In the radial organization, the xylem and phloem alternate. The collateral organization is found in angiosperms and gymnosperms and consists of many vascular bundles, each containing xylem and phloem. It may be open collateral if the procambium is retained between the xylem and phloem, or closed collateral when the meristem is lost. In the bicollateral organization, the xylem is covered by the phloem, which can be found in certain angiosperms. Two types of organization show concentric vascular tissues: the amphivasal (having inner phloem) is often observed in monocots and some dicots, whereas the amphicribal (containing inner xylem) is found in ferns and some aquatic angiosperms.
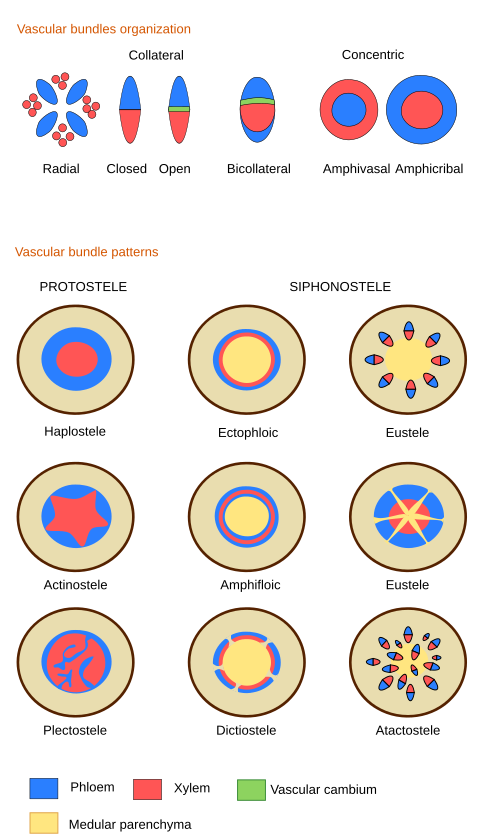
Vascular tissue arrangements that differ from eusteles during the primary growth of organs are collectively known as vascular variants or alternative growth patterns. For instance, some species develop vascular bundles in the medullary parenchyma combined with several rings of procambium that yield rings of vascular tissues. It is known as the polycyclic stele. Some of these species produce phloem within the secondary xylem (see below) organized in the so-called phloematic islands or interxylar phloem. There are other organizations with vascular tissues forming compartments as separate entities along the shoot. These shoots are referred to as composed shoots.
In angiosperm leaves, the xylem is typically toward the adaxial surface (upper) and the phloem toward the abaxial one (lower). The vascular tissues are organized in cords, known as veins, which run parallel in monocots and are branched in dicots.
2. Xylem
The xylem is responsible for transporting and distributing water and mineral nutrients, which are mainly sourced from the roots, throughout the plant body. It also carries organic and signaling molecules. Furthermore, it functions as a tissue for providing mechanical support to the plant body, especially during secondary growth. The wood of trees and some plants is largely xylem.
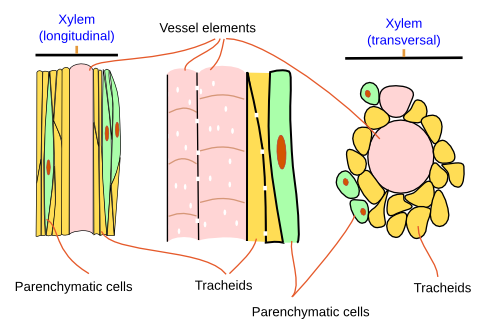
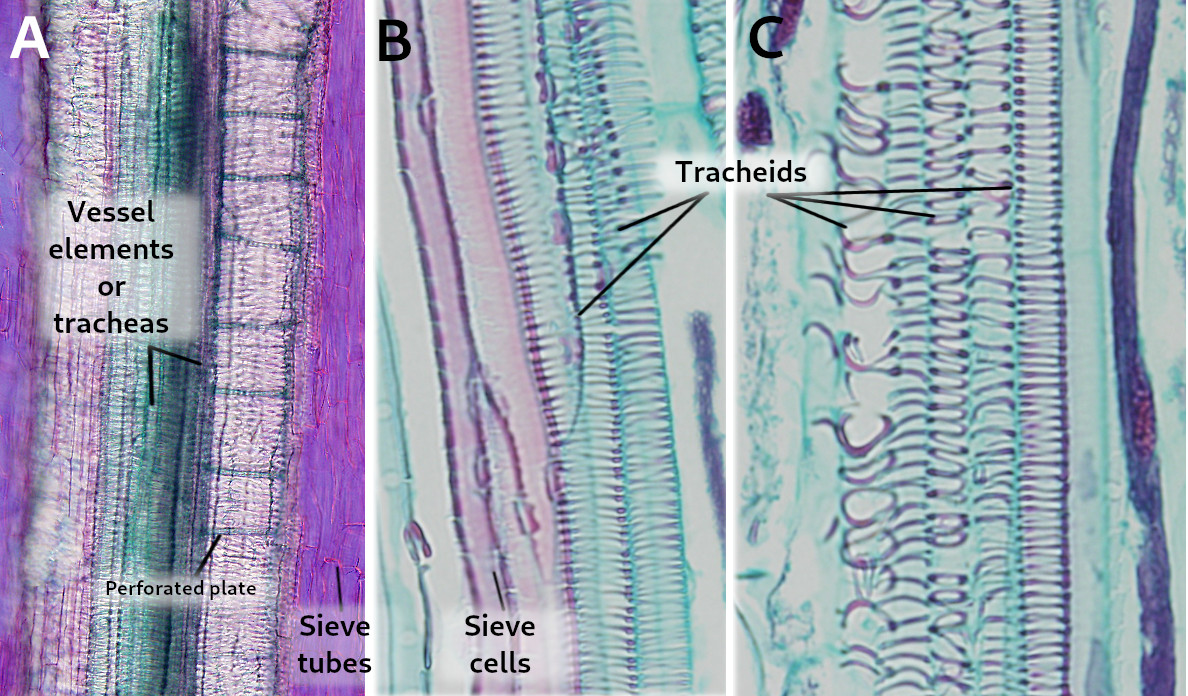
Four main cell types are present in the xylem: a) vessel elements, or tracheae, and b) tracheids are the conducting cells, also known as tracheary elements; c) parenchyma cells work as storage and communication cells; and d) sclerenchyma cells and sclereids are supporting cells (Figure 2).
In gymnosperms, the xylem primarily consists of two cell types: tracheids and parenchyma cells. Tracheids make up 90 % of the xylem, with two main functions: conducting and mechanical support. Parenchyma cells are involved in storage and communicating. In angiosperms, the xylem is more complex. The vessel elements are conducting and supporting cells, although tracheids are also present; sclerenchyma cells and sclereids provide mechanical support; and parenchyma cells serve as storage and communicating cells.
Tracheary elements (cell types a and b) (Figures 2 and 3) are cells containing lignin in their thick and hard secondary cell walls. These cells lose their cytoplasmic content during maturation. The secondary cell wall of the tracheary cells forms distinctive thickenings depending on the cell type. Thus, vessel elements (a) and tracheids (b) are identified at light microscopy by the morphology of these thickenings, which may be annular, helical, scaliform, reticulated, or dotted structures. The annular thickenings are observed in the protoxylem, which are replaced by helical thickenings. Metaxylem shows scaliform, reticulated, and dotted patterns (Figure 4). The scaliform pattern is often observed in pteridophytes but is rare in seed plants. The morphology of the thickenings is determined by the orientation of the cellulose fibers in the cell wall, which in turn depends on the arrangement of the microtubules beneath the plasma membrane. The term "trachea" was given by M. Malpighi because the tracheary elements resemble the respiratory tracheas of insects. The formation of a tracheary element is a process with several steps: a cambium meristematic cell divides periclinally, the new cell grows in length, cellulose and hemicellulose are laid to form the secondary cell wall, lignin is synthesized and released into the cell wall, and regulated cell death occurs. This process is similar in both angiosperms and gymnosperms, but the times and components may differ.

Regulated cell death (apoptosis) happens in many cells throughout plant organs, such as in leaves during senescence, roots during the formation of aerenchyma, flowers during development, and seeds during germination. The degradation of the cell content is fast in the tracheary cells (but slower in sclerenchyma tissues). During xylem differentiation, several factors appear to trigger the process, such as nitric oxide. It is interesting that most cells die after performing their functions, yet the conducting cells of the xylem perform their functions once they are dead. Regulated cell death is a fast process that lasts a few minutes. It begins with a massive influx of calcium and the breakage of the vacuole membrane, leading to the release of many hydrolytic enzymes into the cytoplasm. The previous storage of these enzymes in the vacuole suggests that the process was initiated much earlier. The process of dying runs parallel to the formation of the secondary cell wall. In two days, the cell is empty and ready for conduction.
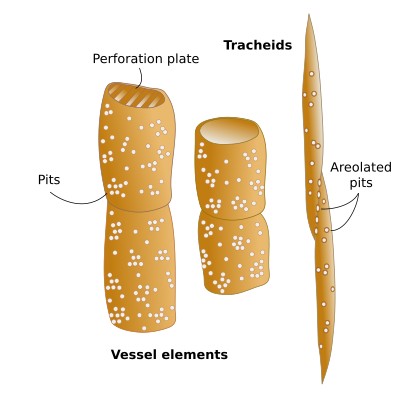
Vessel elements (a) are cells with a larger diameter and flatter ends when compared to tracheids (Figures 2, 3, and 5). They are connected end to end to form long tubes, also known as vessels. Water is conducted via the symplastic pathway, that is, it moves inside the cells. The water moves from one cell to the next through the perforation plates, which are the specialized perforated transverse cell walls at both ends of each cell. Furthermore, water is able to cross the lateral walls of the vessels through pits that form perforated areas and pass to the lateral adjoining cells of the xylem. Vessel elements are the main conducting cells of the xylem of angiosperms. The vessels (the rows) are 1 cm to 1 m long and 15 to 300 µm wide. The vessel elements (the individual conducting cells) may not grow larger than the meristematic cells from which they are differentiated, but the diameter is usually larger.
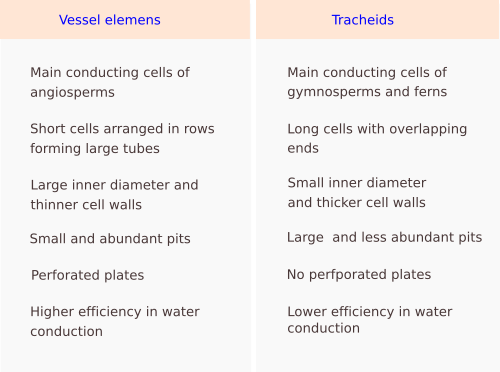
Tracheids (b) are the other conducting cell type of vascular plants. They are elongated, spindle-shaped, and narrow (Figure 3). It is the only tracheary element in pteridophytes and gymnosperms. In gymnosperms, tracheids are up to 90% of the all xylem cells, and, aside from conduction, they are the main mechanical supporting cell. To perform these functions, they have to die. In gymnosperms, tracheids are long and tipped, measuring 6 mm long and 6 to 60 µm wide. In cross sections, they appear round to polygonal. Angiosperms have both tracheids and vessel elements. Water moves from one cell to the next through areolate pits found at the cell walls of both ends of each tracheid, which overlap with those of the previous and following cells, respectively (Figure 3). Water is also transported laterally through areolate pits located at the lateral cell walls. Because tracheids do not have perforation plates, they have a lower capacity for water conduction than vessel elements. Furthermore, tracheids have thicker cell walls and a smaller inner volume for conduction compared to vessel elements. Conifers show tracheids with very large and round areolate pits and an inner structure known as the torus, which is an oval thickening of the cell wall. The torus can regulate the flow of water through the pit.
From an evolutionary point of view, tracheids predate the vessel elements. That is, tracheids were the only type of conducting cell in the first vascular plants, while vessel elements were "invented" later. It is also thought that both the vessel elements for conducting and the sclerenchyma fibers for mechanical support evolved from tracheids. In fact, the more primitive angiosperms have vessel elements that share morphological features with tracheids. In some conifers, tracheids may also work as storage cells. Some current angiosperm groups, such as Winteraceae, have tracheids in the xylem as the only conducting cell type. These tracheids are long cells (4.5 mm) with thin cell walls and blunt ends that overlap with the ends of adjacent tracheids.
Tracheary cells are generated by proliferation and differentiation of cells originating from either the procambium meristem or the vascular cambium meristem. In trees, the cell cycle of a vascular cambium cell is long and may last from 10 to 15 days, depending on the species and environment. Once the new cell is produced, it grows by loosening the cell wall and by the influx of water driven by the inner concentration of substances. In this way, the hydraulic pressure pushes against the cell wall. During cell growth, many molecules need to be synthesized to expand the cell wall. These early processes are crucial, as the cell may enlarge 10 to 100 times its initial size. Besides water availability, cell growth is influenced by phytohormones like auxin, gibberellins, and cytokinins. The secondary cell wall is synthesized and formed after the final size is accomplished. The secondary cell wall is 2 to 10 µm thick, poorly hydrated, and very stiff. It is the main component of wood. It is composed of cellulose (40-60% of the dry weight), hemicellulose (10-40% of the dry weight), and lignin (10 - 35% of the dry weight).
Parenchyma cells (c) are another cell type of the xylem. Commonly, the parenchymatic component is more developed in angiosperms than in gymnosperms. It is about 26% of the xylem in angiosperms and about 6% in conifers. The larger proportion of parenchyma is found in the xylem of succulents and lianas. In addition, temperature seems to be the main factor that promotes the increase of the parenchyma content in the xylem.
Parenchyma cells are arranged in the conducting tissues in two orientations: radially or axially, which are perpendicular to one another. Radial parenchyma cells form rows, or rays, that are perpendicular to the surface of the organ, while axial parenchyma cells form clusters, or rows, arranged longitudinally in the xylem and are more abundant in the secondary xylem (see below). The number and features of axial and radial parenchyma are variable among plant species, and these differences have been used in evolutionary studies. Certain plant species lack radial parenchyma.
Radial parenchyma cells are spindle-shaped, parallel to the axis of the ray, and connected to one another and to other cell types by numerous plasmodesmata that allow the transport of substances. They account for most of the living cells in the xylem. The rays may range from less than 1 mm to several cm in height and from one to several cells in width. They are named uniseriate when there is a single row of cells, biseriate when there are two rows, and multiseriate if there are more than two rows. In some species, several thin rays may merge and look like a single one, known as aggregated rays. In addition, some plants show only one kind of ray, while others have different types of rays. Homocellular rays contain one type of parenchyma cells, whereas rays showing cells with different features are referred to as heterocellular rays. Heterocellular rays are regarded as ancient compared with homocellular rays. In conifers, the rays are either uniseriate or biseriate, whereas in angiosperms, they tend to be multiseriate. The rays of the xylem are continuous with the rays of the phloem. This is due to the fact that one single cell of the cambium meristem can differentiate into radial parenchyma cells of either the xylem or phloem. The phloem rays are usually less easily discerned than the xylem ones.
Axial parenchyma cells are often in contact with conducting cells, with a distribution pattern that depends on the species. However, the axial parenchyma cells are more abundant in the phloem than in the xylem. The axial parenchyma in the xylem is classified as paratracheal when it is associated with the vessel elements and apotracheal when it is not. There are three types of paratracheal parenchyma: vasicentric, when it is in contact with the vessel elements; aliphorm, when it surrounds the vessels and extends away as wings; and confluent, when it forms bands and parenchyma cells surround and contact the vessels. The apotracheal parenchyma can be divided into four types: terminal, when it appears in the last layers of new xylem; limitant, when it appears on both sides of the growing xylem; diffuse, when it is interspersed throughout xylem layers; and banded, when it appears in small bands scattered among the vessels. The apotracheal organization is regarded as primitive compared with paratracheal, which emeged later.
Parenchyma cells perform multiple functions: store carbohydrates (starch), water, nitrogen, and mineral; participate in the defense against pathogens; allow communication between phloem and xylem; cooperate during lignification of tracheary components; refill those conducting cells that get air bubbles inside (cavitation); influence the mechanical properties of the tracheary components; synthesize metabolites and resins, among other functions. For instance, the rays of the xylem can store starch, allow communication between the xylem and phloem, and carry molecules to the vascular cambium, which is very active during some seasons.
Sclerenchyma fibers and sclereids (d) provide support and protection. Xylem sclerenchyma fibers retain their cytoplasm during a period of their active phase, some ot them for a brief period, whereas others remain alive as long as the plant is alive. Sclerenchyma fibers are longer than vessel elements, have a smaller diameter, have relatively thick cell walls and pointed ends. In some primitive species, sclerenchyma fibers look like tracheids and are named as fibro-tracheids. Sclerenchyma fibers may show septa that extend from the cell wall, creating compartments within the cytoplasm to store substances like starch. The mechanical support function of sclerenchyma fibers and sclereids is mostly performed in angiosperms. In gymnosperms, tracheids carry out both mechanical and conducting functions.

The primary xylem is the first type of xylem developed during the formation of a plant organ. First, it is protoxylem, and afterwards, metaxylem. The protoxylem is differentiated from the procambium meristem as the organ grows. The protoxylem fully matures and subsequently disappears due to the compression of mechanical forces that are generated by the growth of the organ. The cell walls of protoxylem conducting cells, vessel elements and tracheids, show annular thickenings first, and then helical thickenings in cells that differentiate later on. Because the protoxylem is the first conducting tissue to be functional, it forms near the apical meristems, and therefore it is the main way to supply water and other substances to these meristems. Protoxylem tracheary cells die to be functional but retain the ability to be extended. They assemble a primary cell wall and ring thickenings as the secondary cell wall, which provides mechanical support but does not resist elongation. These features are also found in cells with helical thickenings in the secondary cell wall. In some parts of the plant, however, the ongoing stretching forces during the organ growth may lead to cell walls and the cells to break. The broken cells of the protoxylem are known as protoxylem lacunae. The metaxylem appears after the protoxylem, developes as the organ enlarges, and matures once the growth begins to slow. It also arises from the procambium meristem. Their cells show larger diameters than those of the protoxylem, and the cell walls of the conducting cells have reticulated thickenings first and perforated thickenings later. The metaxylem is the functional mature xylem in those organs that don't go through secondary growth.
The secondary xylem is produced from the vascular cambium meristem in those organs undergoing secondary growth. Together with secondary phloem, it is the mature conducting tissue in plants with secondary growth. The secondary xylem is divided into a radial and an axial component. The radial component consists of cells organized perpendicular to the organ surface, while the axial component, which constitutes the largest part of the xylem, is organized parallel to the long axis of the organ. The radial component primarily consists of parenchyma cells, with tracheids in some species. In gymnosperms, the axial component is formed of tracheids and parenchyma cells. Many conifers show resin canals within this tissue. In angiosperms, the axial component consists of tracheids, sclerenchyma fibers, vessel elements, and parenchyma cells. This tissue may also display secretory ducts
The xylem transports water, most of which is meant for transpiration. Only about 5% of the water transported by the xylem is used for cell growth, while 1% is ultilized for photosynthesis. Hundreds of molecules of water are transpired for each fixed CO2 molecule during photosynthesis. If plants had evolved a mechanism to absorb CO2 without losing water, their need for water would be drastically reduced. On the other hand, transpiration is an energy-saving process for moving water from the roots to the aerial parts, ultimately being released through stomata, as the water is moved by the water's tension-cohesion property.
Roots inform the aboveground parts of the plant about the underground availability of water and nutrients by using the xylem as the communication pathway. Certain hormones, such as ethylene, abscisic acid, and cytokinins, along with other compounds, are carried by the xylem from the roots to the aerial parts. In addition, these signaling molecules transmit information regarding the association of roots with fungi (mycorrhizae) or bacteria (nodules in legumes). Micronutrients like free amino acids, such as histidine, nicotinamide, and organic acids join iron, copper, nickel, zinc, and manganese. These micronutrients may cross into the phloem, because they have been found there.
3. Phloem
The phloem, also known as sieve tissue or bast, consists of living cells. Its main role to transport and distribute soluble organic molecules, mostly carbohydrates, synthesized by photosynthesis or mobilized from storage tissues. Besides nutrients, the phloem transports signaling molecules, such as peptides, hormones, proteins, and RNAs. It is a system to ship resources to plant organs. The concentration of molecules in the phloem is quite high, and the upload with carbohydrates has to be done against the concentration gradient. The phloem is also a pathway for pathogens to produce a systemic infection of the plant. The production of phloem compared with xylem is higher in gymnosperms than in angiosperms.
The phloem is made up of more cell types than the xylem. There are conducting cells and non-conducting cells. The Conducting cells are the sieve tubes (a) and sieve cells (b) (Figures 6, 7, 8, and 9). Both cell types are living cells, even though they lack a functional nucleus, and their primary cell walls are thickened with callose deposits. Only some Pinaceae develop a secondary cell wall in the conducting cells. During differentiation, the conducting cells undergo a partial and selective autolysis that removes some organelles. Thus, the vacuole membrane breaks, ribosomes, cytoskeleton, and Golgi apparatus cisterns disappear, while the nucleus is partially degraded. Part of the cytoplasm is retained, which includes proteins that associate to form fibers, an anastomosed network of smooth endoplasmic reticulum, modified mitochondria, and some plastids. The cell walls of the cell ends show highly modified plasmodesmata that form the sieve plates.
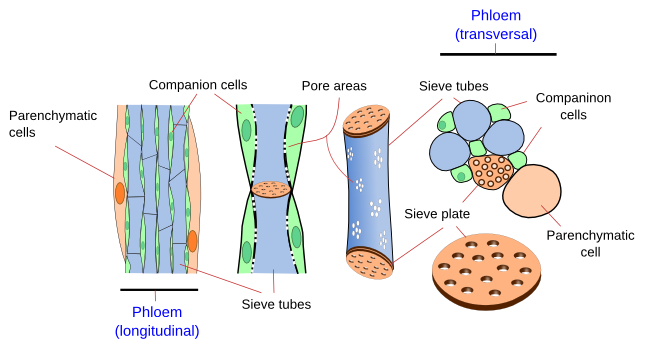
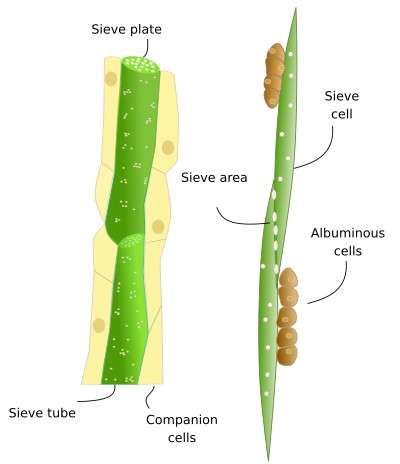
Non-conducting cells are parenchyma cells (c). There is parenchyma cells associated with sieve tubes and with sieve cells. Through asymmetric division, a meristematic cell may differentiate into a conducting cell and its associated parenchyma cell. However, sieve tubes without associated parenchyma cells can also be observed, for instance, in the leaves of grasses and protophloem of roots. There are also supporting cells in the phloem, such as sclerenchyma fibers and sclereids (d).
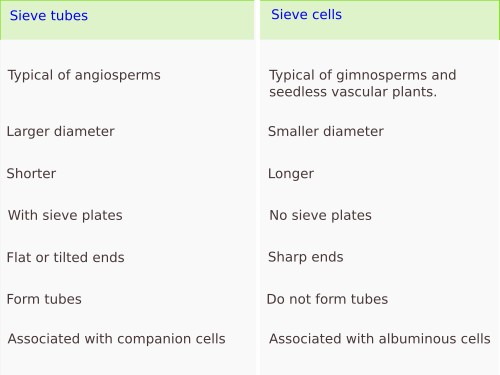
Sieve tubes (a) (Figures 6, 7, 8, and 10) are the typical conducting cells of angiosperms. They are among the most differentiated cells of the plant. Sieve tubes are cells lined up into longitudinal rows that communicate with one another through sieve plates located at both ends of each cell. Sieve plates have large-sized pores that allow the direct connection between adjoining cytoplasms, making up a symplastic system. Sieve plates are formed by the coalescence of plasmodesmata in the fields of pits. They can be compound sieve plates when they are formed from many pit areas, or sieve fields, in more primitive plants, or simple sieve plates when they are formed from a very large sieve field in more modern species. In addition, there are sieve fields with plasmodesmata on the side walls of sieve tubes, with a pore diameter that enlarges as the tissue ages. The lateral sieve fields communicate with adjacent sieve tubes and sieve tubes connect to parenchyma cells, known as companion cells. In angiosperms, many sieve tubes have pores filled with callose deposits. However, it is not known whether these cylinder-like callose deposits serves a purpouse in living cells, as they are present in damaged or non-functional sieve tubes. Occasionally, callose deposits can span the entire sieve area.
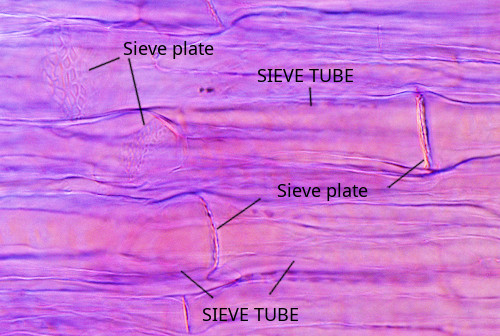
The development of sieve plates takes place during the differentiation of sieve tubes. There are three phases: enucleation, thickening of the cell wall, and formation of the sieve plate. Initially, only the middle lamella of the cell wall separates the two daughter cells. Callose deposits are laid on each side of the middle lamella. The primary cell wall is assembled between two neighboring callose deposits. Finally, the callose deposits get thinner, and the middle lamella is degraded, forming a channel that allows communication between the two cytoplasms. The pore may remain surrounded by a ring of callose or be completely clean.
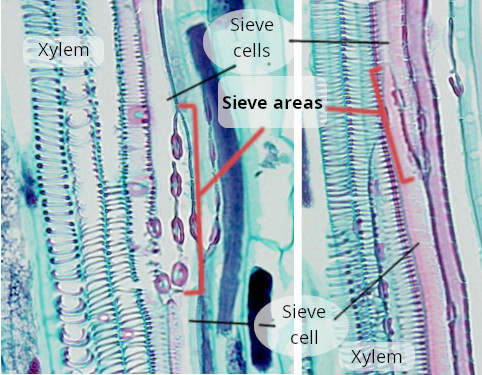
Sieve cells (b) (Figures 7, 8, 9, and 10) are typical of gymnosperms, pteridophytes, and other plants with primitive features. They are long cells with pointed ends, communicating with each other laterally by primary pore fields that form the sieve areas. They don't have sieve plates. Functionally and morphologically, sieve cells are associated with a type of specialized parenchymal cell called Strassburger's cells (albuminous cells). Sieve cells are the only conductive element found in gymnosperms and pteridophytes. Like sieve tubes, sieve cells lose most of their cytoplasmic content during differentiation. Some Pinaceae may contain sieve cells with secondary cell walls.
Parenchyma cells (c) are components of the phloem. They can be closely associated with sieve tubes and sieve cells. Companion cells are parenchyma cells strongly associated with the sieve tubes of angiosperms. This communication is mediated by plasmodesmata. Companion cells maintain the metabolism of sieve tubes. In fact, the functional unit of the angiosperm phloem is the sieve tube/companion cell. Commonly, a companion cell is associated with only one sieve tube. Both cell types are arise from the same meristematic cell. The companion cells keep sieve tubes alive, as well as upload and download the transported organic substances. Companion cells contain a large nucleus and a cytoplasm full of organelles, indicating an elevated metabolic rate. However, they do not store starch. The cytoplasm of companion cells is usually darker than that of sieve tubes. In stems, sieve tubes and companion cells are initially connected with other phloem cells, but plasmodesmata are progressively closed, leaving only those connections between sieve cells and companion cells functional. The size of companion cells is comparatively larger in the uploading phloem of organs, such as leaves and storage organs, compared with sieve tubes in other organs. In the mesophyll of leaves, the companion cells are involved in loading sieve tubes with photosynthesized compounds. They have plasmodesmata, often branched, that are connected to the pores of the side sieve fields. However, both cell types are relatively isolated from the surrounding cells in the conducting phloem in the stems. In the downloading phloem found in organs where the organic compounds are utilized, the companion cells are either lacking or scarce and very small, while the sieve tubes are connected with other neighboring parenchyma cells. Certain species do not have companion cells.
In gymnosperms, the parenchyma cells associated with the sieve cells are known as Strasburger cells, or albuminous cells (c) (Figure 6). Although they have similar functions as companion cells of angiosperms, the origin of Strasburger cells is different. They are mostly radial marginal cells in contact with the sieve cells that show a cytoplasm with many organelles. Strasburger cells are connected to sieve cells through sieve fields/plasmodesmata complexes and with radial parenchyma cells through plasmodesmata.
Other types of parenchyma cells (c) are present in the phloem. Some of them serve as reservoirs for substances transported by the phloem itself. In some species, there are cells in the phloem that are specialized for secretion. In many conifers, large axial parenchyma cells are observed in the secondary phloem.
Sclerenchyma fibers and sclereids (d) are found in the phloem with supporting and protection roles. Sclerenchyma fibers are abundant in the secondary phloem. They are large and pointed cells, often showing thick secondary cell walls.
The primary phloem is the first phloem to be functional in developing organs. The initial form of primary phloem to appear is the protophloem, which develops from the procambium meristem or from the apical meristems. In angiosperms, protophloem shows sieve tubes that are not fully developed, while in gymnosperms and ferns, it shows poorly developed sieve cells. Companion cells are rare or missing and are generated once the protophloem is differentiated. The protophloem is generated before the protoxylem and, therefore, it is closer to the apical meristem. This allows a supply of organic nutrients to the apical meristems. If the protophloem is not fully functional, the apical meristem is not organized properly. Metaphloem replaces protophloem as the development progresses, usually when the organ stops growing. It also arises from the procambium meristem. The metaphloem has sieve tubes, or sieve cells, that are thicker and longer than those found in protophloem. In angiosperms, it always includes companion cells, and Strasbuger cells in gymnosperms. The sieve tubes have sieve plates. Metaphloem is the functional conducting tissue in plants and organs with primary growth.
Morphologically, it is difficult to distinguish the protophloem from the metaphloem. They are named according to when they appear during organ development. Protophloem is present in elongating parts, while metaphloem is in those regions where primary growth ceases. In protophloem, the sieve tubes are usually associated with parenchyma cells that subsequently develop into sclerenchyma fibers, which will form the sclerenchyma sheath around vascular bundles. The companion cells are observed in the metaphloem. In the primary phloem, there are fibers arranged in cords intended to protect the phloem from being crushed by surrounding tissues. However, the primary phloem is compressed during secondary growth and stops being functional.
The secondary phloem arises from the vascular cambium meristem in plants undergoing secondary growth. The conducting cells and associated parenchyma in the secondary phloem resemble those found in the primary phloem. The conducting cells are well-developed, as are the companion cells, and both axial and radial parenchyma are present. The axial parenchyma cells may be difficult to differentiate from the associated parenchyma. Unlike in xylem, secondary phloem cells do not synthesize secondary cell walls, and therefore they are living cells. In the trees, the secondary phloem involved in conducting nutrients is limited, and the sieve tubes live for about a year because they are crushed by the expanding secondary xylem.
In plants, the sap flux within the phloem conducting cells is powered by concentration gradients. This fluid propelling model was suggested by Münch in yje early 20th century. The gradients are enough to boost the conduction even in the tallest trees, although the speed of the sap in these trees may be 10 times slower than in herbaceous plants.
The substances transported through the phloem are vital for plants. The transport is a three-step process: uploading conducting cells in source organs, mostly leaves; transport through the sieve tubes (or sieve cells); and downloading substances in the target tissues. The osmotic gradient is the power that frives the sap along the phloem. As mentioned before, many types of organic molecules are driven through the phloem, such as proteins, RNAs, carbohydrates, and signaling molecules like hormones. In the leaves, these molecules are uploaded through three pathways: apoplastic, active symplastic, and passive symplastic (Figure 12). It is unknown why one of these methods is selected in a particular plant species. In the apoplastic method, the carbonated substances are synthesized in the cells of the leaf mesophyll and move into the cytoplasm of the phloem parenchyma cells by diffusion. Subsequently, the transported molecules are released into the extracellular space (apoplastic) by plasma membrane transporters. The companion cells (in angiosperms) uptake the molecules through plasma membrane transporters, and once inside the cells, the molecules reach the sieve tubes by diffusion. In the active simplastic way, carbohydrates get to the companion cells through plasmodesmata. Then carbohydrates are transformed into oligomers, preventing them from crossing plasmodesmata again, yet they can still diffuse to the sieve tubes because pores communicating companion cells and sieve tubes are larger. In the passive simplastic method, carbonated molecules reach the sieve tubes via a concentration gradient. Aquaporins are water membrane channels that enable these osmotic pressures. The three ways of uploading the phloem may coexist in the same plant or alternate between them depending on the circumstances. Specialized companion cells are required for each uploading method.
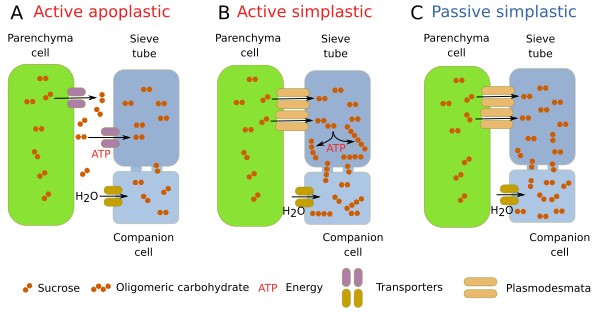
During the daytime, the phloem is uploaded with carbohydrates produced by photosynthesis, while during the nighttime, the uploaded substances come from starch reserves and vacuoles. In addition, the conduction along the phloem is not much affected by transpiration. The speed of transport seems to be quite fast along the stem. The speed of conduction depends on the size of the sieve tubes and the size of the pores in the sieve plates. It is thought that the potential conduction capacity of the phloem is high, and it is more influenced by the download process in the target organs. The energy for conduction is generated by osmosis at the upload regions. In the downloading regions, the output flux is greatly influenced by plasmodesmata that communicate sieve tubes and parenchyma cells.
In the target organs, the molecules transported by the phloem leave sieve cells by passive facilitated diffusion or by carbohydrate transporters, for instance, by carbohydrate/proton symport. In any case, the phloem loses water, since water goes along with organic molecules during downloading. The phloem has to deliver many molecules in many places, and the amount of molecules depends on the demands and state of each target region.
-
Bibliography ↷
-
Furuta KM, Hellmann E, Helariutta Y. 2014. Molecular control of cell specification and cell differentiation during procambial development. Annual review of plant biology. 65:607-638. DOI: annurev-arplant-050213-040306.
Lalonde S, Francesch VR, Frommer WB. 2001. Commpanion cells. In: eLS. John Wiley & Sons Ltd, Chichester. DOI: 10.1038/npg.els.0002087.
Liesche J, Patrick J. 2017. An update on phloem transport: a simple bulk flow under complex regulation. F1000Research 6(F1000 Faculty Rev): 2096. DOI: 10.12688/f1000research.12577.1
Lucas WL, Groover A, Lichtenberger R, Furuta K, Yadav S-R, Helariutta Y, He X-Q, Fukuda H, Kang J, Brady SM, Patrick JW, Sperry J, Yoshida A, López-Millán A-F, Grusak MA, Kachroo P. 2013. The Plant Vascular System: Evolution, Development and Functions. Journal of integrative plant biology. 55: 294–388. DOI:10.1111/jipb.12041.
Scarpella E, Meijer AH. 2004. Pattern formation in the vascular system of monocot and dicot plant species. New phytologist. 164: 209-242. DOI: 10.1111/j.1469-8137.2004.01191.x.
Spicer R. 2014. Symplasmic network in secondary vascular tissues: parenchyma distribution and activity supporting long-distant transport. Journal of experimental botany. 65: 1829-1848. DOI: 10.1093/jxb/ert459.
-
 Support
Support