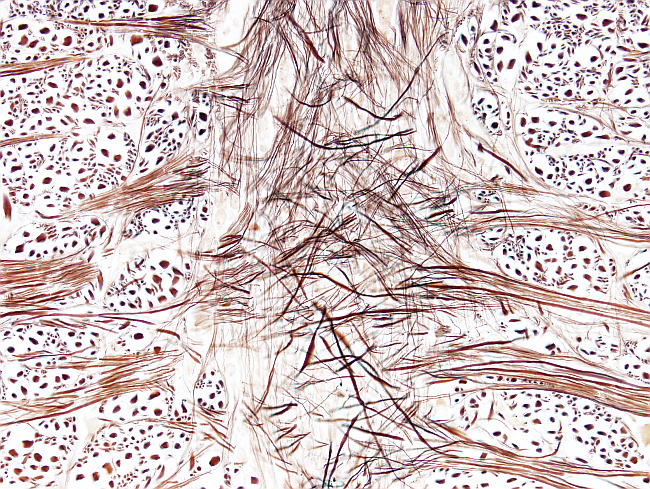
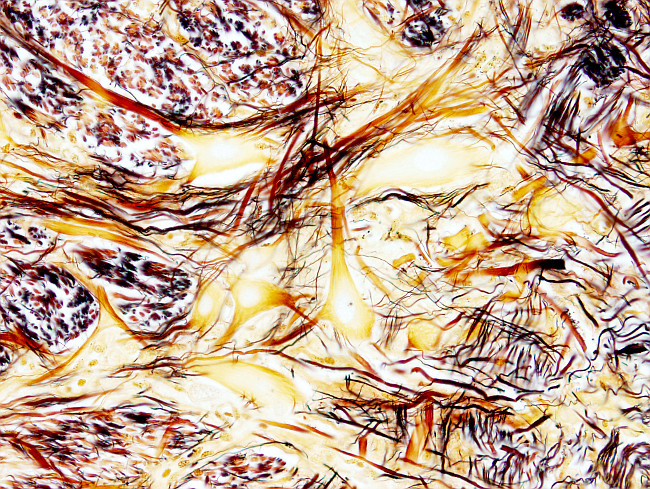
The silver impregnation procedure is intended for labeling the nervous fibers and bundles, as well as cell bodies of neurons. The protocol is done on several mm thick blocks obtained from brain or spinal cord.
Procedure
In this technique, the fixative composition is important.
1. Fixation by immersion. 16-18 h at 4-10 ºC.
Fixative:
4 g Chloral hydrat
20 ml100º ethanol
25 ml distilled H2O
Adjust pH to 8.6 with ammonia
2. 100º ethanol 24 h.
3. 1.5 % silver nitrate in distilled H2O for 5 days at 37 ºC in darkness.
4. Rinse twice, 30 s each, in distilled H2O.
5. Reduction. 24 h at room temperature.
0.5 g Hydroquinone
40 ml distilled H2O
3.5 ml of formaldehyde 40 %
6. 70º ethenol 3 x 1 min.
7. The tissue blocks are embedded in paraffin (see protocol ↗).
8. 10 µm thick sections.
9. Remove paraffin and hydrate sections. No more thant 5 mn in each ethanol.
The sections can be stained with hematoxylin after hydration to label the nuclei of neurons and glia, so the fiber bundles are observed in their tissue context.
10. Dehydration in ethanol, xylene, mounting and coversliped.
Dehydration has to be quick, no more than five min in each step. The mounting medium may affect the impregnation. Better results are obtained by embedding the samples in epoxy-like resins (and propylene oxide as intermediary liquid). It means a last polymerization step at 60 ºC for 24 h.
Results
Neurons: weak brown.
Fibers (axons): black. brwon.
Notes
The results are quite variable, and it is difficult to obtain standard outputs. Good quality products give better results.
The time in the reduction solution is variable. It is stopped depending on the overall color of the tissue block.
It is not advisable to use tissue blocks larger than 1 cm.
Paraffin removing, dehydration and hydration have to be quick.
Products
50º, 70º, 80º, 90º, 96º y 100º ethanol
Chloral hydrate
Silver nitrate
Distilled H2O
Ammonia
Hydroquinone
Fornaldehyde
Propilene oxide
Xylene
Paraffin
Epoxy-like resin
Labware
Containers and vials
Stove 60 º
Stove 37 º